Phytomedicine ( IF 6.7 ) Pub Date : 2021-08-17 , DOI: 10.1016/j.phymed.2021.153719 Guanghong Chen 1 , Honglin Xu 1 , Yuting Wu 1 , Xin Han 1 , Lingpeng Xie 1 , Guoyong Zhang 1 , Bin Liu 2 , YingChun Zhou 1
|
Background
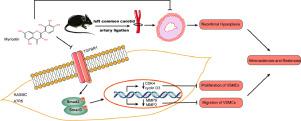
Neointimal formation, mediated by the proliferation and migration of vascular smooth muscle cells (VSMCs), is a common pathological basis for atherosclerosis and restenosis. Myricetin, a natural flavonoid, reportedly exerts anti-atherosclerotic effects. However, the effect and mechanism of myricetin on VSMCs proliferation and migration and neointimal hyperplasia (NIH) remain unknown.
Purpose
We investigated myricetin's effect on NIH, as well as the potential involvement of transforming growth factor-beta receptor 1 (TGFBR1) signaling in mediating myricetin's anti-atherosclerotic and anti-restenotic actions.
Methods
Myricetin's effects on the proliferation and migration of HASMCs and A7R5 cells were determined by CCK-8, EdU assays, wound healing, Transwell assays, and western blotting (WB).Molecular docking, molecular dynamics (MD) simulation, surface plasmon resonance (SPR) and TGFBR1 kinase activity assays were employed to investigate the interaction between myricetin and TGFBR1. An adenovirus vector encoding TGFBR1 was used to verify the effects of myricetin. In vivo, the left common carotid artery (LCCA) ligation mouse model was adopted to determine the impacts of myricetin on neointimal formation and TGFBR1 activation.
Results
Myricetin dose-dependently inhibited the migration and proliferation in VSMCs, suppressed the expression of CDK4, cyclin D3, MMP2, and MMP9. Molecular docking revealed that myricetin binds to key regions for TGFBR1 antagonist binding, and the binding energy was -9.61 kcal/mol. MD simulation indicated stable binding between TGFBR1 and myricetin. Additionally, SPR revealed an equilibrium dissociation constant of 4.35 × 10−5 M between myricetin and TGFBR1. According to the TGFBR1 kinase activity assay, myricetin directly inhibited TGFBR1 kinase activity (IC50 = 8.551 μM). Furthermore, myricetin suppressed the phosphorylation level of TGFBR1, Smad2, and Smad3 in a dose-dependent pattern, which was partially inhibited by TGFBR1 overexpression. Consistently, TGFBR1 overexpression partially rescued the suppressive roles of myricetin on VSMCs migration and proliferation. Moreover, myricetin dramatically inhibited NIH and reduced TGFBR1, Smad2, and Smad3 phosphorylation in the LCCA.
Conclusion
This is the first study to demonstrate that myricetin suppresses NIH and VSMC proliferation and migration via inhibiting TGFBR1 signaling. Myricetin can be developed as a potential therapeutic candidate for treating atherosclerosis and vascular restenosis.
中文翻译:

杨梅素通过抑制 TGFBR1 信号通路抑制血管平滑肌细胞增殖和迁移并抑制新生内膜增生
背景
由血管平滑肌细胞 (VSMC) 的增殖和迁移介导的新内膜形成是动脉粥样硬化和再狭窄的常见病理基础。据报道,杨梅素是一种天然黄酮类化合物,具有抗动脉粥样硬化作用。然而,杨梅素对血管平滑肌细胞增殖、迁移和新生内膜增生(NIH)的作用和机制尚不清楚。
目的
我们调查了杨梅素对 NIH 的影响,以及转化生长因子 β 受体 1 (TGFBR1) 信号传导在介导杨梅素抗动脉粥样硬化和抗再狭窄作用中的潜在作用。
方法
杨梅素对 HASMC 和 A7R5 细胞增殖和迁移的影响通过 CCK-8、EdU 检测、伤口愈合、Transwell 检测和蛋白质印迹 (WB) 确定。 分子对接、分子动力学 (MD) 模拟、表面等离子体共振 (SPR) ) 和 TGFBR1 激酶活性测定用于研究杨梅素和 TGFBR1 之间的相互作用。使用编码 TGFBR1 的腺病毒载体来验证杨梅素的作用。在体内,采用左颈总动脉(LCCA)结扎小鼠模型来确定杨梅素对新内膜形成和 TGFBR1 激活的影响。
结果
杨梅素剂量依赖性地抑制 VSMC 的迁移和增殖,抑制 CDK4、细胞周期蛋白 D3、MMP2 和 MMP9 的表达。分子对接显示杨梅素与TGFBR1拮抗剂结合的关键区域结合,结合能为-9.61 kcal/mol。MD 模拟表明 TGFBR1 和杨梅素之间的稳定结合。此外,SPR 显示杨梅素和 TGFBR1 之间的平衡解离常数为 4.35 × 10 -5 M。根据 TGFBR1 激酶活性测定,杨梅素直接抑制 TGFBR1 激酶活性 (IC 50= 8.551 微米)。此外,杨梅素以剂量依赖性模式抑制 TGFBR1、Smad2 和 Smad3 的磷酸化水平,这部分被 TGFBR1 过表达抑制。一致地,TGFBR1 过表达部分挽救了杨梅素对 VSMC 迁移和增殖的抑制作用。此外,杨梅素显着抑制 NIH 并降低 LCCA 中的 TGFBR1、Smad2 和 Smad3 磷酸化。
结论
这是第一项证明杨梅素通过抑制 TGFBR1 信号传导抑制 NIH 和 VSMC 增殖和迁移的研究。杨梅素可被开发为治疗动脉粥样硬化和血管再狭窄的潜在治疗候选药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号