Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2021-08-17 , DOI: 10.1016/j.jinorgbio.2021.111578 Kaitlyn Frankenfield 1 , Darya Marchany-Rivera 2 , Kayla G Flanders 3 , Anthony Cruz-Balberdy 4 , Juan Lopez-Garriga 2 , Jose F Cerda 3
|
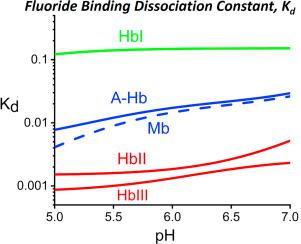
The studies on the L. pectinata hemoglobins (HbI, HbII, and HbIII) are essential because of their biological roles in hydrogen sulfide transport and metabolism. Variation in the pH could also play a role in the transport of hydrogen sulfide by HbI and oxygen by HbII and HbIII, respectively. Here, fluoride binding was used to further understand the structural properties essential for the molecular mechanism of ligand stabilization as a function of pH. The data allowed us to gain insights into how the physiological roles of HbI, HbII, HbIII, adult hemoglobin (A-Hb), and horse heart myoglobin (Mb) have an impact on the heme-bound fluoride stabilization. In addition, analysis of the vibrational assignments of the met-cyano heme complexes shows varied strength interactions of the heme-bound ligand. The heme pocket composition properties differ between HbI (GlnE7 and PheB10) and HbII/HbIII (GlnE7 and TyrB10). Also, the structural GlnE7 stereo orientation changes between HbI and HbII/HbIII. In HbI, its carbonyl group orients towards the heme iron, while in HbII/HbIII, the amino group occupies this position. Therefore, in HbI, the interactions to the heme-bound fluoride ion, cyanide, and oxygen with GlnE7 via H-bonding are not probable. Still, the aromatic cage PheB10, PheCD1, and PheE11 may contribute to the observed stabilization. However, a robust H-bonding networking stabilizes HbII and HbIII, heme-bound fluoride, cyanide, and oxygen ligand with the OH and NH2 groups of TyrB10 and GlnE7, respectively. At the same time, A-Hb and Mb have moderate but similar ligand interactions controlled by their respective distal E7 histidine.
中文翻译:

氟化物与特征血红素口袋中心的结合:深入了解配体稳定性
对梳状乳杆菌血红蛋白(HbI、HbII 和 HbIII)的研究至关重要,因为它们在硫化氢转运和代谢中发挥着生物学作用。 pH 值的变化也可能分别在 HbI 运输硫化氢和 HbII 和 HbIII 运输氧气方面发挥作用。在这里,氟化物结合用于进一步了解配体稳定的分子机制所必需的结构特性(作为 pH 的函数)。这些数据使我们能够深入了解 HbI、HbII、HbIII、成人血红蛋白 (A-Hb) 和马心肌红蛋白 (Mb) 的生理作用如何影响血红素结合氟化物的稳定性。此外,对间氰基血红素复合物的振动分配的分析显示了血红素结合配体的不同强度的相互作用。 HbI(GlnE7 和 PheB10)和 HbII/HbIII(GlnE7 和 TyrB10)之间的血红素袋成分特性不同。此外,结构 GlnE7 立体定向在 HbI 和 HbII/HbIII 之间发生变化。在 HbI 中,其羰基朝向血红素铁,而在 HbII/HbIII 中,氨基占据该位置。因此,在 HbI 中,血红素结合的氟离子、氰化物和氧与 GlnE7 不可能通过氢键相互作用。尽管如此,芳香笼 PheB10、PheCD1 和 PheE11 可能有助于观察到的稳定性。然而,强大的氢键网络可分别通过 TyrB10 和 GlnE7 的 OH 和 NH 2基团稳定 HbII 和 HbIII、血红素结合的氟化物、氰化物和氧配体。同时,A-Hb 和 Mb 具有中等但相似的配体相互作用,由各自的远端 E7 组氨酸控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号