Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2021-08-17 , DOI: 10.1016/j.jorganchem.2021.122026 Anna Wieczorek-Błauż 1 , Andrzej Błauż 2 , Błażej Rychlik 2 , Damian Plażuk 1
|
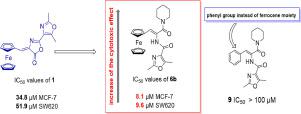
We described a synthesis of new 3-ferrocenylpropenamides prepared by oxazolone-ring opening reaction with various primary and secondary amines. The antiproliferative activities of the obtained compounds were screened on human tumor cell lines (HCT116, SW620, Colo 205, MCF-7, HepG2 and A549). We found that the newly prepared compounds showed cytotoxicity in micromolar range, with the most active piperidinyl derivative 6b with IC50 values 8.1 μM (MCF-7), 9.6 μM (SW620) and 13.2 μM (Colo 205). Cell cycle analysis showed that 6b increases number of G2/M arrested cells.
中文翻译:

5(4H)-恶唑酮衍生的 3-二茂铁基丙烯酰胺的合成和生物活性
我们描述了通过与各种伯胺和仲胺的恶唑酮开环反应制备的新型 3-二茂铁基丙烯酰胺的合成。在人类肿瘤细胞系(HCT116、SW620、Colo 205、MCF-7、HepG2 和 A549)上筛选所得化合物的抗增殖活性。我们发现新制备的化合物在微摩尔范围内显示出细胞毒性,其中最活跃的哌啶衍生物6b的 IC 50值为 8.1 μM (MCF-7)、9.6 μM (SW620) 和 13.2 μM (Colo 205)。细胞周期分析表明6b增加了G 2 /M停滞细胞的数量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号