Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2021-08-16 , DOI: 10.1016/j.jcou.2021.101678 Ruoyu Zhang 1 , Anlu Wei 1 , Min Zhu 1 , Xiaoxia Wu 1 , Hua Wang 1 , Xinli Zhu 1 , Qingfeng Ge 2
|
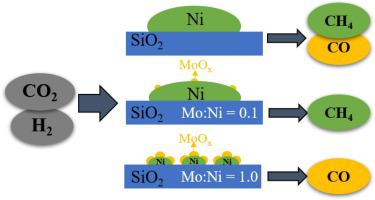
Catalytic reduction of CO2 to CO via reverse water gas shift (RWGS) reaction provides a feasible approach to utilize CO2, since CO can be further converted to various versatile products through the syngas routes. Ni-based catalysts are low cost and have a high activity for CO2 reduction but are nonselective for RWGS due to competition from methanation. In this work, we demonstrated that surface modification of Ni by MoOx can be tailored to tune the reactions of RWGS and methanation. The addition of MoOx improves Ni dispersion through strong interactions whereas partially reduced MoOx modifies the surface of Ni particles through both geometric coverage and electronic modification. No CO adsorption was observed at room temperature on the NiMo catalyst with a Mo/Ni ratio of 1, confirmed by density functional theory calculation. Tracking product evolution showed that CO2 is first reduced to CO through RWGS on the Ni catalysts and methane is a product of CO hydrogenation. Apparent activation energy analysis indicates that the overall reaction is controlled by CO desorption. Addition of a small amount of Mo (Mo/Ni ratio of 0.1) shifts the reaction further to methanation selective with ∼100% CH4 selectivity as MoOx aids in the activation of both CO2 and CO. In contrast, the addition of a large amount of Mo (Mo/Ni ratio of 1) shifts the reaction to RWGS selective with a CO selectivity > 94%. This is attributed to the enhanced CO desorption from the surface as a result of MoOx modification.
中文翻译:

通过 MoOx 表面改性调节 Ni 催化剂上 CO2 还原过程中的反向水煤气变换和甲烷化反应
通过反向水煤气变换 (RWGS) 反应将 CO 2催化还原为 CO提供了一种利用 CO 2的可行方法,因为 CO 可以通过合成气路线进一步转化为各种通用产品。镍基催化剂成本低,对 CO 2还原具有高活性,但由于甲烷化的竞争,对 RWGS 没有选择性。在这项工作中,我们证明了 MoO x对 Ni 的表面改性可以调整以调整 RWGS 和甲烷化的反应。MoO x的添加通过强相互作用改善了 Ni 的分散性,而部分减少了 MoO x通过几何覆盖和电子修饰来修饰 Ni 颗粒的表面。通过密度泛函理论计算证实,在室温下,在 Mo/Ni 比为 1 的 NiMo 催化剂上没有观察到 CO 吸附。跟踪产物演化表明,CO 2首先通过镍催化剂上的 RWGS 还原为 CO,而甲烷是 CO 加氢的产物。表观活化能分析表明整个反应受 CO 解吸控制。添加少量 Mo(Mo/Ni 比为 0.1)将反应进一步转移到甲烷化选择性,CH 4选择性约为 100%,因为 MoO x有助于两种 CO 2的活化和 CO。相比之下,添加大量 Mo(Mo/Ni 比为 1)将反应转变为 RWGS 选择性,CO 选择性 > 94%。这归因于 MoO x改性导致从表面增强的 CO 解吸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号