当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Differential Geminal Substitution of γ Amino Acid Residues at the (i + 2) Position of αγ Turn Segments on the Conformation of Template β-Hairpin Peptides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-16 , DOI: 10.1021/acs.joc.1c00351 Swapna Debnath 1 , Suvankar Ghosh 2 , Gopal Pandit 1 , Priyadarshi Satpati 2 , Sunanda Chatterjee 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-16 , DOI: 10.1021/acs.joc.1c00351 Swapna Debnath 1 , Suvankar Ghosh 2 , Gopal Pandit 1 , Priyadarshi Satpati 2 , Sunanda Chatterjee 1
Affiliation
|
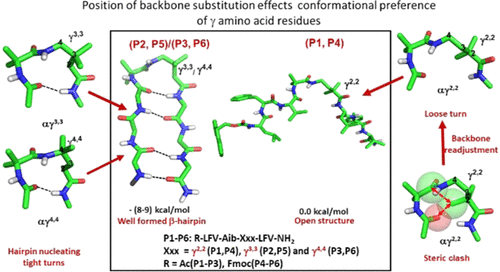
The effect of insertion of three geminally dimethyl substituted γ amino acid residues [γ2,2 (4-amino-2,2-dimethylbutanoic acid), γ3,3 (4-amino-3,3-dimethylbutanoic acid), and γ4,4 (4-amino-4,4-dimethylbutanoic acid)] at the (i + 2) position of a two-residue αγ C12 turn segment in a model octapeptide sequence Leu-Phe-Val-Aib-Xxx-Leu-Phe-Val (where Xxx = γ amino acid residues) has been investigated in this study. Solution conformational studies (NMR, CD, and IR) and ab initio calculations indicated that γ3,3 and γ4,4 residues were well accommodated in the β-hairpin nucleating αγ C12 turns, which gave rise to well-registered hairpins, in contrast to γ2,2, which was unable to form a tight C12 β-hairpin nucleating turn and promote a well-registered β-hairpin. Geminal disubstitution at the Cα carbon in γ2,2 led to unfavorable steric contacts, disabling its accommodation in the αγ C12 hairpin nucleating turn unlike the γ3,3 and γ4,4 residues. Geminal substitutions at different carbons along the backbone constrained backbone torsion angles for the three γ amino acid residues differently, generating diverse conformational preferences in them. Folded hairpins were energetically more stable (∼8 to 9 kcal/mol) than the unfolded peptides. Conformational preference of the peptides was independent of the N-terminal protecting group. Such fundamental understanding will instrumentalize the future directed design of foldamers.
中文翻译:

在αγ转角片段的(i + 2)位置γ氨基酸残基的差异双生取代对模板β-发夹肽构象的影响
插入三个双二甲基取代的 γ 氨基酸残基 [γ 2,2 (4-氨基-2,2-二甲基丁酸)、γ 3,3 (4-氨基-3,3-二甲基丁酸) 和 γ 的影响4,4 (4-氨基-4,4-二甲基丁酸)] 在模型八肽序列 Leu-Phe-Val-Aib-Xxx-Leu中的两个残基 αγ C 12转角区段的 ( i + 2) 位置-Phe-Val(其中 Xxx = γ 氨基酸残基)已在本研究中进行了研究。溶液构象研究(NMR、CD 和 IR)和从头计算表明 γ 3,3和 γ 4,4残基很好地容纳在 β-发夹成核 αγ C 12 中与γ 2,2形成鲜明对比的是,γ 2,2不能形成紧密的C 12 β-发夹成核转角并促进良好记录的β-发夹。与γ 3,3和γ 4,4不同,γ 2,2 中C α碳处的成对置换导致不利的空间接触,使其无法适应αγ C 12发夹形核转角残留物。沿主链的不同碳原子处的双生替换以不同方式限制了三个 γ 氨基酸残基的主链扭转角,从而在其中产生了不同的构象偏好。折叠的发夹在能量上比未折叠的肽更稳定(~8 到 9 kcal/mol)。肽的构象偏好不依赖于 N 端保护基团。这种基本的理解将有助于未来的折叠器定向设计。
更新日期:2021-09-03
中文翻译:

在αγ转角片段的(i + 2)位置γ氨基酸残基的差异双生取代对模板β-发夹肽构象的影响
插入三个双二甲基取代的 γ 氨基酸残基 [γ 2,2 (4-氨基-2,2-二甲基丁酸)、γ 3,3 (4-氨基-3,3-二甲基丁酸) 和 γ 的影响4,4 (4-氨基-4,4-二甲基丁酸)] 在模型八肽序列 Leu-Phe-Val-Aib-Xxx-Leu中的两个残基 αγ C 12转角区段的 ( i + 2) 位置-Phe-Val(其中 Xxx = γ 氨基酸残基)已在本研究中进行了研究。溶液构象研究(NMR、CD 和 IR)和从头计算表明 γ 3,3和 γ 4,4残基很好地容纳在 β-发夹成核 αγ C 12 中与γ 2,2形成鲜明对比的是,γ 2,2不能形成紧密的C 12 β-发夹成核转角并促进良好记录的β-发夹。与γ 3,3和γ 4,4不同,γ 2,2 中C α碳处的成对置换导致不利的空间接触,使其无法适应αγ C 12发夹形核转角残留物。沿主链的不同碳原子处的双生替换以不同方式限制了三个 γ 氨基酸残基的主链扭转角,从而在其中产生了不同的构象偏好。折叠的发夹在能量上比未折叠的肽更稳定(~8 到 9 kcal/mol)。肽的构象偏好不依赖于 N 端保护基团。这种基本的理解将有助于未来的折叠器定向设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号