当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen-Initiated Free-Radical Polymerization of Alkyl Acrylates at High Temperatures
Macromolecules ( IF 5.1 ) Pub Date : 2021-08-16 , DOI: 10.1021/acs.macromol.1c00669 Shi Liu 1, 2, 3 , Lauren Chua 3 , Ahmad Arabi Shamsabadi 4 , Patrick Corcoran 4 , Abhirup Patra 3 , Michael C. Grady 5 , Masoud Soroush 4 , Andrew M. Rappe 3
Macromolecules ( IF 5.1 ) Pub Date : 2021-08-16 , DOI: 10.1021/acs.macromol.1c00669 Shi Liu 1, 2, 3 , Lauren Chua 3 , Ahmad Arabi Shamsabadi 4 , Patrick Corcoran 4 , Abhirup Patra 3 , Michael C. Grady 5 , Masoud Soroush 4 , Andrew M. Rappe 3
Affiliation
|
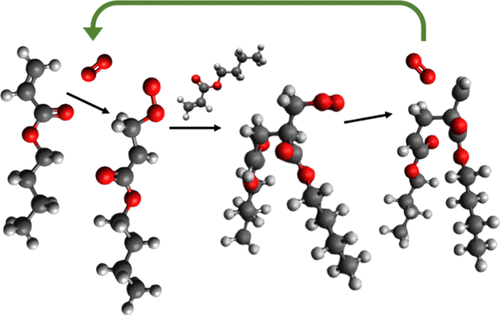
Molecular oxygen has been reported to be a strong inhibitor at low temperatures. Here, it is shown that the introduction of oxygen gas into the free-radical polymerization of n-butyl acrylate (nBA) results in high conversion of the monomer in a very short time at temperatures above 140 °C without the use of any conventional initiators. By conducting first-principles calculations based on density functional theory, this work for the first time identifies and kinetically studies the most-likely reactions via which molecular oxygen contributes to polymer chain initiation. It provides theoretical and experimental evidence that molecular oxygen acts as a catalyst in alkyl acrylate free-radical polymerization at high temperatures. A triplet diradical intermediate is generated from solvated oxygen reacting with an alkyl acrylate monomer. The intermediate then reacts with another monomer and thermally dissociates from molecular oxygen to proceed toward polymerization. This theoretical finding is supported by laboratory experiments showing that in the presence of a very small amount of molecular oxygen and in the absence of any thermal initiators, free-radical polymerization of nBA occurs sustainably and proceeds to a very high monomer conversion. This work opens a new path for a more economic and sustainable production of higher-quality acrylic polymers using molecular oxygen as an initiator catalyst.
中文翻译:

烷基丙烯酸酯在高温下的氧引发自由基聚合
据报道,分子氧在低温下是一种强抑制剂。在这里,表明在丙烯酸正丁酯的自由基聚合中引入氧气(nBA) 在 140 °C 以上的温度下在很短的时间内实现单体的高转化率,而无需使用任何常规引发剂。通过基于密度泛函理论进行第一性原理计算,这项工作首次识别并动力学研究分子氧有助于聚合物链引发的最可能的反应。它提供了理论和实验证据,证明分子氧在高温下作为丙烯酸烷基酯自由基聚合的催化剂。三线态双自由基中间体由溶剂化氧与丙烯酸烷基酯单体反应生成。然后中间体与另一种单体反应并从分子氧中热解离以进行聚合。n BA 可持续发生并进行非常高的单体转化。这项工作为使用分子氧作为引发剂催化剂更经济、更可持续地生产更高质量的丙烯酸聚合物开辟了一条新途径。
更新日期:2021-09-14
中文翻译:

烷基丙烯酸酯在高温下的氧引发自由基聚合
据报道,分子氧在低温下是一种强抑制剂。在这里,表明在丙烯酸正丁酯的自由基聚合中引入氧气(nBA) 在 140 °C 以上的温度下在很短的时间内实现单体的高转化率,而无需使用任何常规引发剂。通过基于密度泛函理论进行第一性原理计算,这项工作首次识别并动力学研究分子氧有助于聚合物链引发的最可能的反应。它提供了理论和实验证据,证明分子氧在高温下作为丙烯酸烷基酯自由基聚合的催化剂。三线态双自由基中间体由溶剂化氧与丙烯酸烷基酯单体反应生成。然后中间体与另一种单体反应并从分子氧中热解离以进行聚合。n BA 可持续发生并进行非常高的单体转化。这项工作为使用分子氧作为引发剂催化剂更经济、更可持续地生产更高质量的丙烯酸聚合物开辟了一条新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号