当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd-Catalyzed Direct Diversification of Natural Anti-Alzheimer’s Disease Drug: Synthesis and Biological Evaluation of N-Aryl Huperzine A Analogues
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-08-16 , DOI: 10.1021/acs.jnatprod.1c00600 Shi-Xing Miao 1 , Lin-Xi Wan 1 , Zhen-Xiang He 1 , Xian-Li Zhou 1 , Xiaohuan Li 1 , Feng Gao 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-08-16 , DOI: 10.1021/acs.jnatprod.1c00600 Shi-Xing Miao 1 , Lin-Xi Wan 1 , Zhen-Xiang He 1 , Xian-Li Zhou 1 , Xiaohuan Li 1 , Feng Gao 1
Affiliation
|
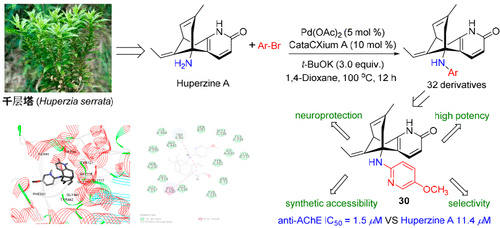
The first systematic direct diversification of a complex natural product by metal-catalyzed N–H functionalization was carried out. A new series of N-(hetero)aryl analogues (1–32) of the natural anti-Alzheimer’s disease drug huperzine A (HPA) was prepared via palladium-catalyzed Buchwald–Hartwig cross-coupling reactions of HPA with various aryl bromides in good yields. Most of the N-aryl-huperzine A (N-aryl-HPA) analogues showed good acetylcholinesterase (AChE) inhibitory activity in in vitro experiments. Three arylated huperzine A analogues (14, 19, and 30) exhibited stronger anti-AChE activity than HPA. The 5-methoxy-2-pyridyl analogue (30) displayed the most potent AChE inhibition activity, with an IC50 value of 1.5 μM, which was 7.6-fold more active than HPA. Compound 30 also exhibited better neuroprotective activity for H2O2-induced damage in SH-SY5Y cells than HPA. Structure–activity relationship analysis suggested that the electron density of the installed aromatic ring or heteroaromatic ring played a significant role in inducing the AChE inhibition activity. Overall, compound 30 showed the advantages of easy synthesis, high potency and selectivity, and improved neuroprotection, making it a potential huperzine-type lead compound for Alzheimer’s disease drug development.
中文翻译:

天然抗阿尔茨海默病药物的钯催化直接多样化:N-芳基石杉碱甲类似物的合成和生物学评价
通过金属催化的 N-H 功能化对复杂天然产物进行了首次系统的直接多样化。一系列新的ñ - (杂)芳基类似物(1 - 32)的天然抗阿尔茨海默氏病药物石杉碱甲(HPA)的经HPA的与良好的各种芳基溴化物的钯催化的Buchwald-Hartwig的交叉偶联反应制备产量。大多数N-芳基-石杉碱甲 ( N-芳基-HPA) 类似物在体外实验中显示出良好的乙酰胆碱酯酶 (AChE) 抑制活性。三个芳基化石杉碱甲类似物(14,19,和30) 表现出比 HPA 更强的抗 AChE 活性。5-甲氧基-2-吡啶基类似物 ( 30 ) 显示出最有效的 AChE 抑制活性,IC 50值为 1.5 μM,比 HPA 的活性高 7.6 倍。与HPA相比,化合物30对SH-SY5Y细胞中H 2 O 2诱导的损伤也表现出更好的神经保护活性。构效关系分析表明,安装的芳环或杂芳环的电子密度在诱导AChE抑制活性中起重要作用。总体而言,化合物30 显示出易于合成、高效能和选择性以及改善神经保护等优点,使其成为用于阿尔茨海默病药物开发的潜在石杉碱型先导化合物。
更新日期:2021-08-27
中文翻译:

天然抗阿尔茨海默病药物的钯催化直接多样化:N-芳基石杉碱甲类似物的合成和生物学评价
通过金属催化的 N-H 功能化对复杂天然产物进行了首次系统的直接多样化。一系列新的ñ - (杂)芳基类似物(1 - 32)的天然抗阿尔茨海默氏病药物石杉碱甲(HPA)的经HPA的与良好的各种芳基溴化物的钯催化的Buchwald-Hartwig的交叉偶联反应制备产量。大多数N-芳基-石杉碱甲 ( N-芳基-HPA) 类似物在体外实验中显示出良好的乙酰胆碱酯酶 (AChE) 抑制活性。三个芳基化石杉碱甲类似物(14,19,和30) 表现出比 HPA 更强的抗 AChE 活性。5-甲氧基-2-吡啶基类似物 ( 30 ) 显示出最有效的 AChE 抑制活性,IC 50值为 1.5 μM,比 HPA 的活性高 7.6 倍。与HPA相比,化合物30对SH-SY5Y细胞中H 2 O 2诱导的损伤也表现出更好的神经保护活性。构效关系分析表明,安装的芳环或杂芳环的电子密度在诱导AChE抑制活性中起重要作用。总体而言,化合物30 显示出易于合成、高效能和选择性以及改善神经保护等优点,使其成为用于阿尔茨海默病药物开发的潜在石杉碱型先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号