当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acetylation of the Catalytic Lysine Inhibits Kinase Activity in PI3Kδ
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-08-16 , DOI: 10.1021/acschembio.1c00225 Julie C L Fournier 1, 2 , John P Evans 3 , Francesca Zappacosta 4 , Daniel A Thomas 3 , Vipulkumar K Patel 1 , Gemma V White 1 , Sebastien Campos 5 , Nicholas C O Tomkinson 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-08-16 , DOI: 10.1021/acschembio.1c00225 Julie C L Fournier 1, 2 , John P Evans 3 , Francesca Zappacosta 4 , Daniel A Thomas 3 , Vipulkumar K Patel 1 , Gemma V White 1 , Sebastien Campos 5 , Nicholas C O Tomkinson 2
Affiliation
|
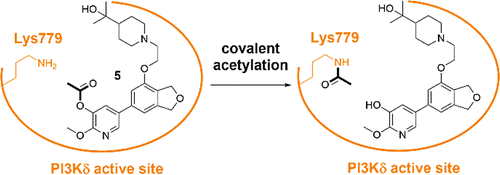
Covalent inhibition is a powerful strategy to develop potent and selective small molecule kinase inhibitors. Targeting the conserved catalytic lysine is an attractive method for selective kinase inactivation. We have developed novel, selective inhibitors of phosphoinositide 3-kinase δ (PI3Kδ) which acylate the catalytic lysine, Lys779, using activated esters as the reactive electrophiles. The acylating agents were prepared by adding the activated ester motif to a known selective dihydroisobenzofuran PI3Kδ inhibitor. Three esters were designed, including an acetate ester which was the smallest lysine modification evaluated in this work. Covalent binding to the enzyme was characterized by intact protein mass spectrometry of the PI3Kδ-ester adducts. An enzymatic digest coupled with tandem mass spectrometry identified Lys779 as the covalent binding site, and a biochemical activity assay confirmed that PI3Kδ inhibition was a direct result of covalent lysine acylation. These results indicate that a simple chemical modification such as lysine acetylation is sufficient to inhibit kinase activity. The selectivity of the compounds was evaluated against lipid kinases in cell lysates using a chemoproteomic binding assay. Due to the conserved nature of the catalytic lysine across the kinome, we believe the covalent inhibition strategy presented here could be applicable to a broad range of clinically relevant targets.
中文翻译:

催化赖氨酸的乙酰化抑制 PI3Kδ 中的激酶活性
共价抑制是开发有效和选择性小分子激酶抑制剂的有力策略。靶向保守的催化赖氨酸是一种有吸引力的选择性激酶失活方法。我们开发了新型选择性磷酸肌醇 3-激酶 δ (PI3Kδ) 抑制剂,其使用活化酯作为反应性亲电试剂,将催化赖氨酸 Lys779 酰化。通过将活化的酯基序加入已知的选择性二氢异苯并呋喃 PI3Kδ 抑制剂来制备酰化剂。设计了三种酯,包括醋酸酯,这是本工作中评估的最小赖氨酸修饰。通过 PI3Kδ-酯加合物的完整蛋白质质谱法表征与酶的共价结合。酶解与串联质谱联用将 Lys779 鉴定为共价结合位点,生化活性测定证实 PI3Kδ 抑制是共价赖氨酸酰化的直接结果。这些结果表明简单的化学修饰如赖氨酸乙酰化足以抑制激酶活性。使用化学蛋白质组学结合测定针对细胞裂解物中的脂质激酶评估化合物的选择性。由于跨激酶组的催化赖氨酸的保守性质,我们相信这里提出的共价抑制策略可适用于广泛的临床相关靶标。这些结果表明简单的化学修饰如赖氨酸乙酰化足以抑制激酶活性。使用化学蛋白质组学结合测定针对细胞裂解物中的脂质激酶评估化合物的选择性。由于跨激酶组的催化赖氨酸的保守性质,我们相信这里提出的共价抑制策略可适用于广泛的临床相关靶标。这些结果表明简单的化学修饰如赖氨酸乙酰化足以抑制激酶活性。使用化学蛋白质组学结合测定针对细胞裂解物中的脂质激酶评估化合物的选择性。由于跨激酶组的催化赖氨酸的保守性质,我们相信这里提出的共价抑制策略可适用于广泛的临床相关靶标。
更新日期:2021-09-17
中文翻译:

催化赖氨酸的乙酰化抑制 PI3Kδ 中的激酶活性
共价抑制是开发有效和选择性小分子激酶抑制剂的有力策略。靶向保守的催化赖氨酸是一种有吸引力的选择性激酶失活方法。我们开发了新型选择性磷酸肌醇 3-激酶 δ (PI3Kδ) 抑制剂,其使用活化酯作为反应性亲电试剂,将催化赖氨酸 Lys779 酰化。通过将活化的酯基序加入已知的选择性二氢异苯并呋喃 PI3Kδ 抑制剂来制备酰化剂。设计了三种酯,包括醋酸酯,这是本工作中评估的最小赖氨酸修饰。通过 PI3Kδ-酯加合物的完整蛋白质质谱法表征与酶的共价结合。酶解与串联质谱联用将 Lys779 鉴定为共价结合位点,生化活性测定证实 PI3Kδ 抑制是共价赖氨酸酰化的直接结果。这些结果表明简单的化学修饰如赖氨酸乙酰化足以抑制激酶活性。使用化学蛋白质组学结合测定针对细胞裂解物中的脂质激酶评估化合物的选择性。由于跨激酶组的催化赖氨酸的保守性质,我们相信这里提出的共价抑制策略可适用于广泛的临床相关靶标。这些结果表明简单的化学修饰如赖氨酸乙酰化足以抑制激酶活性。使用化学蛋白质组学结合测定针对细胞裂解物中的脂质激酶评估化合物的选择性。由于跨激酶组的催化赖氨酸的保守性质,我们相信这里提出的共价抑制策略可适用于广泛的临床相关靶标。这些结果表明简单的化学修饰如赖氨酸乙酰化足以抑制激酶活性。使用化学蛋白质组学结合测定针对细胞裂解物中的脂质激酶评估化合物的选择性。由于跨激酶组的催化赖氨酸的保守性质,我们相信这里提出的共价抑制策略可适用于广泛的临床相关靶标。









































 京公网安备 11010802027423号
京公网安备 11010802027423号