Brain Research ( IF 2.7 ) Pub Date : 2021-08-14 , DOI: 10.1016/j.brainres.2021.147622 Lifang Wu 1 , Qiang Du 2 , Congcong Wu 3
|
Background
Alzheimer’s Disease (AD) is a neurodegenerative disorder characterized by progressive memory loss and cognition and language impairment. CircRNA lysophosphatidic acid receptor 1 (circLPAR1) was found to be increased in AD patients, however, the potential role of circLPAR1 in AD process remains unclear.
Methods
Beta-amyloid (Aβ) 25–35-stimulated CHP-212 and IMR-32 cells were used to perform expression and function analyses. The expression of genes and proteins was determined by qRT-PCR and Western blot. Cell proliferation and apoptosis were analyzed using cell counting kit-8 (CCK-8) assay, flow cytometry, and Western blot, respectively. ELISA analysis was used to detect the levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α). The levels of reactive oxygen species (ROS), lactate dehydrogenase (LDH) and superoxide dismutase (SOD) were detected using commercial kits. The direct interactions between miR-212-3p and ZNF217 (Zinc finger protein 217) or circLPAR1 was verified using dual-luciferase reporter and RNA immunoprecipitation (RIP) assays.
Results
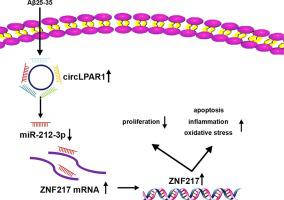
CircLPAR1 was highly expressed in AD patients and Aβ25-35-stimulated CHP-212 and IMR-32 cells. Knockdown of circLPAR1 suppressed Aβ25-35-induced neuronal apoptosis, inflammation, and oxidative stress. Mechanistically, circLPAR1 competitively bound to miR-212-3p to elevate its target ZNF217. Rescue experiments suggested that miR-212-3p inhibition reversed circLPAR1 silencing-evoked inhibition on neuronal injury under Aβ25-35 stimulation. Moreover, miR-212-3p re-expression reduced Aβ25-35-induced neuronal apoptosis, inflammation, and oxidative stress, which were abolished by ZNF217 up-regulation.
Conclusion
CircLPAR1 promotes Aβ25-35-induced apoptosis, inflammation, and oxidative stress via miR-212-3p/ZNF217 axis, suggesting a new insight into the pathogenesis of AD.
中文翻译:

CircLPAR1/miR-212-3p/ZNF217反馈环促进淀粉样蛋白β诱导的阿尔茨海默病神经元损伤
背景
阿尔茨海默病 (AD) 是一种神经退行性疾病,其特征是进行性记忆丧失以及认知和语言障碍。发现 circRNA 溶血磷脂酸受体 1 (circLPAR1) 在 AD 患者中增加,然而 circLPAR1 在 AD 过程中的潜在作用仍不清楚。
方法
β-淀粉样蛋白 (Aβ) 25-35 刺激的 CHP-212 和 IMR-32 细胞用于进行表达和功能分析。通过qRT-PCR和Western印迹确定基因和蛋白质的表达。分别使用细胞计数试剂盒 8 (CCK-8) 测定、流式细胞术和蛋白质印迹分析细胞增殖和凋亡。ELISA分析用于检测白细胞介素(IL)-1β、IL-6和肿瘤坏死因子-α(TNF-α)的水平。使用商业试剂盒检测活性氧 (ROS)、乳酸脱氢酶 (LDH) 和超氧化物歧化酶 (SOD) 的水平。使用双荧光素酶报告基因和 RNA 免疫沉淀 (RIP) 分析验证了 miR-212-3p 和 ZNF217(锌指蛋白 217)或 circLPAR1 之间的直接相互作用。
结果
CircLPAR1 在 AD 患者和 Aβ25-35 刺激的 CHP-212 和 IMR-32 细胞中高表达。circLPAR1 的敲低抑制了 Aβ25-35 诱导的神经元凋亡、炎症和氧化应激。从机制上讲,circLPAR1 与 miR-212-3p 竞争性结合以提升其靶标 ZNF217。救援实验表明 miR-212-3p 抑制逆转了 circLPAR1 沉默诱发的 Aβ25-35 刺激下对神经元损伤的抑制。此外,miR-212-3p 的重新表达减少了 Aβ25-35 诱导的神经元凋亡、炎症和氧化应激,而这些被 ZNF217 上调所消除。
结论
CircLPAR1 通过 miR-212-3p/ZNF217 轴促进 Aβ25-35 诱导的细胞凋亡、炎症和氧化应激,这表明对 AD 发病机制的新认识。









































 京公网安备 11010802027423号
京公网安备 11010802027423号