Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 4.2 ) Pub Date : 2021-08-15 , DOI: 10.1016/j.bbadis.2021.166246 Aysha Dilna 1 , K V Deepak 1 , Nandini Damodaran 1 , Claudia S Kielkopf 2 , Katarina Kagedal 2 , Karin Ollinger 2 , Sangeeta Nath 1
|
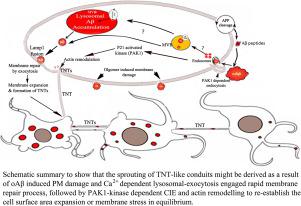
Alzheimer's disease (AD) pathology progresses gradually via anatomically connected brain regions. Direct transfer of amyloid-β1–42 oligomers (oAβ) between connected neurons has been shown, however, the mechanism is not fully revealed. We observed formation of oAβ induced tunneling nanotubes (TNTs)-like nanoscaled f-actin containing membrane conduits, in differentially differentiated SH-SY5Y neuronal models. Time-lapse images showed that oAβ propagate from one cell to another via TNT-like structures. Preceding the formation of TNT-like conduits, we detected oAβ−induced plasma membrane (PM) damage and calcium-dependent repair through lysosomal-exocytosis, followed by massive endocytosis to re-establish the PM. Massive endocytosis was monitored by an influx of the membrane-staining dye TMA-DPH and PM damage was quantified by propidium iodide influx in the absence of Ca2+. The massive endocytosis eventually caused accumulation of internalized oAβ in Lamp1 positive multivesicular bodies/lysosomes via the actin cytoskeleton remodulating p21-activated kinase1 (PAK1) dependent endocytic pathway. Three-dimensional quantitative confocal imaging, structured illumination superresolution microscopy, and flowcytometry quantifications revealed that oAβ induces activation of phospho-PAK1, which modulates the formation of long stretched f-actin extensions between cells. Moreover, the formation of TNT-like conduits was inhibited by preventing PAK1-dependent internalization of oAβ using the small-molecule inhibitor IPA-3, a highly selective cell-permeable auto-regulatory inhibitor of PAK1. The present study reveals that the TNT-like conduits are probably instigated as a consequence of oAβ induced PM damage and repair process, followed by PAK1 dependent endocytosis and actin remodeling, probably to maintain cell surface expansion and/or membrane tension in equilibrium.
中文翻译:

淀粉样蛋白-β 诱导的膜损伤通过 p21 活化激酶依赖性肌动蛋白再调节激发隧道纳米管样导管
阿尔茨海默病 (AD) 病理学通过解剖学上连接的大脑区域逐渐发展。淀粉样蛋白-β 1 – 42寡聚体 (oAβ) 在连接的神经元之间的直接转移已经显示出来,但是,该机制尚未完全揭示。我们在差异分化的 SH-SY5Y 神经元模型中观察到 oAβ 诱导的隧道纳米管 (TNT) 样纳米级 f-肌动蛋白的形成。延时图像显示 oAβ 通过 TNT 样结构从一个细胞传播到另一个细胞。在形成 TNT 样导管之前,我们检测到 oAβ -通过溶酶体胞吐作用诱导质膜 (PM) 损伤和钙依赖性修复,然后进行大量胞吞作用以重建 PM。大量内吞作用通过膜染色染料 TMA-DPH 的流入来监测,PM 损伤通过在没有 Ca 2+的情况下碘化丙锭流入来量化. 大量内吞最终通过肌动蛋白细胞骨架重新调节 p21 活化激酶 1 (PAK1) 依赖的内吞途径,导致 Lamp1 阳性多泡体/溶酶体中内化的 oAβ 积累。三维定量共聚焦成像、结构化照明超分辨率显微镜和流式细胞术定量表明,oAβ 诱导磷酸化 PAK1 的激活,从而调节细胞之间长拉伸 f-肌动蛋白延伸的形成。此外,通过使用小分子抑制剂 IPA-3(PAK1 的高度选择性细胞渗透性自动调节抑制剂)阻止 oAβ 的 PAK1 依赖性内化,抑制了 TNT 样导管的形成。目前的研究表明,TNT 样导管可能是由于 oAβ 诱导的 PM 损伤和修复过程而引起的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号