Inorganic Chemistry Communications ( IF 4.4 ) Pub Date : 2021-08-13 , DOI: 10.1016/j.inoche.2021.108839 Xinyi Yang 1 , Tomohito Kameda 1 , Yuko Saito 1 , Shogo Kumagai 1 , Toshiaki Yoshioka 1
|
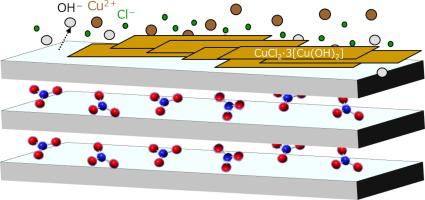
Layered double hydroxides (LDHs) are effective in removing metal cations from aqueous solutions, but the mechanism of the process has not been thoroughly investigated. In this study, we conduct the removal of Cu(II) from aqueous solutions using the CO3·Mg-Al LDH and investigate the mechanism of the process based on equilibrium and pH studies and solid-state analyses. Adsorption isotherms show that the equilibrium removal of Cu follows the Freundlich and Halsey models (R2 = 0.973), inducing a multilayer adsorption process. pH studies show that Cu is removed at pH < pHPZC(=12.1), consistent with chemical binding, rather than electrostatic attraction, as the predominant mechanism of removal. XRD reveals the formation of CuCl2·3[Cu(OH)2] and retention of the LDH structure, and SEM images show a thick-layer morphology. Solid-state analyses confirm the formation of CuCl2·3[Cu(OH)2] and its multilayer accumulation on the LDH surface, in accordance with the equilibrium and pH studies.
中文翻译:

使用插入碳酸盐的 Mg-Al 层状双氢氧化物去除 Cu(II) 的机理研究:平衡和 pH 研究以及固态分析
层状双氢氧化物 (LDH) 可有效去除水溶液中的金属阳离子,但该过程的机制尚未得到彻底研究。在本研究中,我们使用 CO 3 ·Mg-Al LDH从水溶液中去除 Cu(II),并基于平衡和 pH 研究以及固态分析研究该过程的机理。吸附等温线表明,Cu 的平衡去除遵循 Freundlich 和 Halsey 模型(R 2 = 0.973),诱导多层吸附过程。pH 研究表明,Cu 在 pH < pH PZC (=12.1) 时被去除,这与化学结合一致,而不是静电吸引,这是去除的主要机制。XRD 揭示了 CuCl 2 ·3[Cu(OH)2 ]和LDH结构的保留,SEM图像显示出厚层形态。根据平衡和 pH 研究,固态分析证实了 CuCl 2 ·3[Cu(OH) 2 ] 的形成及其在 LDH 表面的多层积累。











































 京公网安备 11010802027423号
京公网安备 11010802027423号