Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2021-08-14 , DOI: 10.1016/j.jinorgbio.2021.111579 Giovanna De Simone 1 , Alessandra di Masi 1 , Paola Fattibene 2 , Chiara Ciaccio 3 , Carlos Platas-Iglesias 4 , Massimo Coletta 3 , Alessandra Pesce 5 , Paolo Ascenzi 6
|
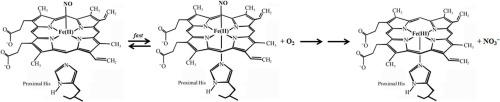
The O2-mediated oxidation of all-β-barrel ferrous nitrosylated nitrobindin from Arabidopsis thaliana (At-Nb(II)-NO), Mycobacterium tuberculosis (Mt-Nb(II)-NO), and Homo sapiens (Hs-Nb(II)-NO) to ferric derivative (At-Nb(III), Mt-Nb(III), and Hs-Nb(III), respectively) has been investigated at pH 7.0 and 20.0 °C. Unlike ferrous nitrosylated horse myoglobin, human serum heme-albumin and human hemoglobin, the process in Nb(II)-NO is mono-exponential and linearly dependent on the O2 concentration, displaying a bimolecular behavior, characterized by kon = (6.3 ± 0.8) × 103 M−1 s−1, (1.4 ± 0.2) × 103 M−1 s−1, and (3.9 ± 0.5) × 103 M−1 s−1 for At-Nb(II)-NO, Mt-Nb(II)-NO, and Hs-Nb(II)-NO, respectively. No intermediate is detected, indicating that the O2 reaction with Nb(II)-NO is the rate-limiting step and that the subsequent conversion of the heme-Fe(III)-N(O)OO− species (i.e., N-bound peroxynitrite to heme-Fe(III)) to heme-Fe(III) and NO3− is much faster. A similar mechanism can be invoked for ferrous nitrosylated human neuroglobin and rabbit hemopexin, in which the heme-Fe(III)-N(O)OO− species is formed as well, although the rate-limiting step seems represented by the reshaping of the six-coordinated heme-Fe(III) complex. Although At-Nb(II)-NO and Mt-Nb(II)-NO are partially (while Hs-Nb(II)-NO is almost completely) penta-coordinated, density functional theory (DFT) calculations rule out that the cleavage of the proximal heme-Fe-His bond in Nb(II)-NO is responsible for the more stable heme-Fe(III)-N(O)OO− species. Moreover, the oxidation of the penta-coordinated heme-Fe(II)-NO adduct does not depend on O2 binding at the proximal side of the metal center. These features may instead reflect the peculiarity of Nb folding and of the heme environment, with a reduced steric constraint for the formation of the heme-Fe(III)-N(O)OO− complex.
中文翻译:

亚硝基化硝基结合素亚铁的氧介导氧化
O 2介导的拟南芥( At -Nb(II)-NO)、结核分枝杆菌( Mt -Nb(II)-NO) 和智人( Hs -Nb( II)-NO) 到铁衍生物(分别为At -Nb(III)、Mt -Nb(III) 和Hs -Nb(III))已在 pH 7.0 和 20.0 °C 下进行了研究。与亚硝基化马肌红蛋白、人血清血红素白蛋白和人血红蛋白不同,Nb(II)-NO 中的过程是单指数的并且线性依赖于 O 2浓度,表现出双分子行为,其特征是k on = (6.3 ± 0.8) × 10 3 M -1 s -1 , (1.4 ± 0.2) × 10 3 M -1 s -1和 (3.9 ± 0.5) × 10 3 M -1 s -1对于At分别为 -Nb(II)-NO、Mt -Nb(II)-NO 和Hs -Nb(II)-NO。没有检测到中间体,表明 O 2与 Nb(II)-NO 的反应是限速步骤,血红素-Fe(III)-N(O)OO -物质(即N-将过氧亚硝酸盐与血红素-Fe(III)) 结合到血红素-Fe(III) 和 NO 3 -快得多。亚硝基化的人神经红蛋白和兔血红素结合蛋白也可以采用类似的机制,其中也形成了血红素-Fe(III)-N(O)OO -物种,尽管限速步骤似乎表现为六配位血红素-Fe(III)配合物。虽然At -Nb(II)-NO 和Mt -Nb(II)-NO 部分(而Hs -Nb(II)-NO 几乎完全)五配位,但密度泛函理论 (DFT) 计算排除了解理Nb(II)-NO 中的近端血红素-Fe-His 键的形成负责更稳定的血红素-Fe(III)-N(O)OO -物种。此外,五配位血红素-Fe(II)-NO加合物的氧化不依赖于O 2结合在金属中心的近侧。这些特征可能反而反映了 Nb 折叠和血红素环境的特殊性,减少了血红素-Fe(III)-N(O)OO -复合物形成的空间约束。











































 京公网安备 11010802027423号
京公网安备 11010802027423号