Experimental Eye Research ( IF 3.0 ) Pub Date : 2021-08-13 , DOI: 10.1016/j.exer.2021.108729 Mackenzie D Martin 1 , Dustin J E Huard 1 , Ricardo C Guerrero-Ferreira 2 , Ishani M Desai 1 , Brett M Barlow 1 , Raquel L Lieberman 1
|
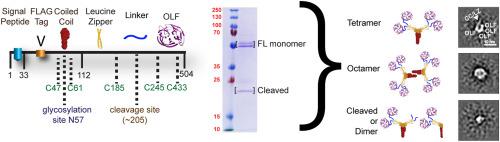
Myocilin, a modular multidomain protein, is expressed broadly in the human body but is best known for its presence in the trabecular meshwork extracellular matrix, and myocilin misfolding is associated with glaucoma. Despite progress in comprehending the structure and misfolding of the myocilin olfactomedin domain, the structure and function of full-length myocilin, and contextual changes in glaucoma, remain unknown. Here we expressed and purified milligram-scale quantities of full-length myocilin from suspension mammalian cell culture (Expi293F), enabling molecular characterization in detail not previously accessible. We systematically characterized disulfide-dependent and -independent oligomerization as well as confirmed glycosylation and susceptibility to proteolysis. We identified oligomeric states with glycosylation sites that are inaccessible to enzymatic removal. Low-resolution single particle 2D class averaging from conventional transmission electron microscopy imaging confirms an extended arrangement of tetramers, truncated products consistent with dimers, and a higher-ordered state consistent with octamer. Taken together, our study reveals new myocilin misfolded states and layers of intrinsic heterogeneity, expands our knowledge of olfactomedin-family proteins and lays the foundation for a better molecular understanding of myocilin structure and its still enigmatic biological function.
中文翻译:

全长肌纤蛋白的分子结构和修饰
Myocilin 是一种模块化的多结构域蛋白,在人体中广泛表达,但最为人所知的是它存在于小梁网细胞外基质中,并且 myocilin 错误折叠与青光眼有关。尽管在理解 myocilin 嗅觉蛋白结构域的结构和错误折叠方面取得了进展,但全长 myocilin 的结构和功能以及青光眼的背景变化仍然未知。在这里,我们从悬浮哺乳动物细胞培养 (Expi293F) 中表达和纯化了毫克级数量的全长肌纤蛋白,从而实现了以前无法获得的详细分子表征。我们系统地表征了依赖二硫键和不依赖二硫键的寡聚化以及确认的糖基化和对蛋白水解的敏感性。我们确定了具有酶去除无法进入的糖基化位点的寡聚状态。来自传统透射电子显微镜成像的低分辨率单粒子 2D 类平均证实了四聚体的扩展排列、与二聚体一致的截断产物以及与八聚体一致的高阶状态。总之,我们的研究揭示了新的肌纤蛋白错误折叠状态和内在异质性层,扩展了我们对嗅觉蛋白家族蛋白的认识,并为更好地理解肌纤蛋白结构及其仍然神秘的生物学功能奠定了基础。和与八聚体一致的高阶状态。总之,我们的研究揭示了新的肌纤蛋白错误折叠状态和内在异质性层,扩展了我们对嗅觉蛋白家族蛋白的认识,并为更好地理解肌纤蛋白结构及其仍然神秘的生物学功能奠定了基础。和与八聚体一致的高阶状态。总之,我们的研究揭示了新的肌纤蛋白错误折叠状态和内在异质性层,扩展了我们对嗅觉蛋白家族蛋白的认识,并为更好地理解肌纤蛋白结构及其仍然神秘的生物学功能奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号