Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioseparation of mandelic acid and substituted derivatives by high-performance liquid chromatography with hydroxypropyl-β-cyclodextrin as chiral mobile additive and evaluation of inclusion complexes by molecular dynamics
Chirality ( IF 2.8 ) Pub Date : 2021-08-14 , DOI: 10.1002/chir.23348 Jie-Hua Shi 1 , Zhen-Yi Lin 1 , Song-Bo Kou 1 , Bao-Li Wang 1 , Shao-Liang Jiang 1
Chirality ( IF 2.8 ) Pub Date : 2021-08-14 , DOI: 10.1002/chir.23348 Jie-Hua Shi 1 , Zhen-Yi Lin 1 , Song-Bo Kou 1 , Bao-Li Wang 1 , Shao-Liang Jiang 1
Affiliation
|
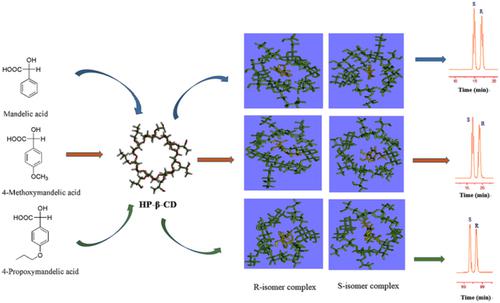
The enantioseparation and resolution mechanism of mandelic acid (MA), 4-methoxymandelic acid (MMA), and 4-propoxymandelic acid (PMA) were investigated by reversed-phase high-performance liquid chromatography (HPLC) with 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) as a chiral mobile-phase additive and molecular dynamics simulation. The suitable chromatographic conditions for the enantioseparation of MA, MMA, and PMA were obtained. Under the selected chromatographic conditions, these enantiomers could achieve baseline separation. The results of thermodynamic parameter analysis revealed that the main driven forces for the enantioseparation of MA, MMA, and PMA could be van der Waals forces and hydrogen-bonding interactions and the chromatographic retention of these chiral compounds was an enthalpy-driven process. The results of the molecular simulation revealed that their chiral resolution mechanism on HP-β-CD was responsible for the formation of inclusion complexes of enantiomers with HP-β-CD with different conformations and binding energies. And the binding energy of HP-β-CD with (S)-isomer was larger than that with (R)-isomer, which is consistent with the experimental results of the first elution of (S)-isomer. Additionally, it is also confirmed that the interaction energies included the van der Waals energy (∆Evdw), electrostatic energy (∆Eelec), polar solvation energy, and SASA energy (∆Esasa), and the separation factor (α) was closely connected with the disparity in the binding energies of optical isomers and HP-β-CD complexes. Meanwhile, from molecular dynamics simulation, it can be found that the ∆(∆Ebinding), (∆(∆Ebinding) = ∆Ebinding,R − ∆Ebinding,S) value was in order of MA–HP-β-CD complex > MMA–HP-β-CD complex > PMA–HP-β-CD complex, which was consistent with the order of Δ(ΔG) values obtained from van't Hoff plot. This indicated that the molecular dynamics simulation has predictive function for chiral resolution.
中文翻译:

以羟丙基-β-环糊精为手性流动添加剂的扁桃酸及其取代衍生物的对映分离及包合物的分子动力学评价
使用 2-羟丙基-β-环糊精的反相高效液相色谱 (HPLC) 研究了扁桃酸 (MA)、4-甲氧基扁桃酸 (MMA) 和 4-丙氧基扁桃酸 (PMA) 的对映分离和拆分机制(HP-β-CD) 作为手性流动相添加剂和分子动力学模拟。获得了适用于 MA、MMA 和 PMA 对映分离的色谱条件。在选定的色谱条件下,这些对映异构体可以实现基线分离。热力学参数分析结果表明,MA、MMA和PMA对映分离的主要驱动力可能是范德华力和氢键相互作用,这些手性化合物的色谱保留是一个焓驱动的过程。分子模拟结果表明,它们对HP-β-CD的手性拆分机制是形成对映异构体与具有不同构象和结合能的HP-β-CD的包合物的原因。HP-β-CD 的结合能与 (S )-异构体大于( R )-异构体,这与( S )-异构体第一次洗脱的实验结果一致。此外,还证实了相互作用能包括范德华能(∆ E vdw)、静电能(∆ E elec)、极性溶剂化能和 SASA 能(∆ E sasa),以及分离因子(α)与旋光异构体和 HP-β-CD 复合物结合能的差异密切相关。同时,由分子动力学模拟可知,Δ( ΔE binding ), (Δ(Δ E binding) = ∆ E binding , R − ∆ E binding , S ) 值的顺序为 MA–HP-β-CD 复合物 > MMA–HP-β-CD 复合物 > PMA–HP-β-CD 复合物,这与从 van't Hoff 图中获得的 Δ(Δ G ) 值的顺序。这表明分子动力学模拟具有手性分辨率的预测功能。
更新日期:2021-09-17
中文翻译:

以羟丙基-β-环糊精为手性流动添加剂的扁桃酸及其取代衍生物的对映分离及包合物的分子动力学评价
使用 2-羟丙基-β-环糊精的反相高效液相色谱 (HPLC) 研究了扁桃酸 (MA)、4-甲氧基扁桃酸 (MMA) 和 4-丙氧基扁桃酸 (PMA) 的对映分离和拆分机制(HP-β-CD) 作为手性流动相添加剂和分子动力学模拟。获得了适用于 MA、MMA 和 PMA 对映分离的色谱条件。在选定的色谱条件下,这些对映异构体可以实现基线分离。热力学参数分析结果表明,MA、MMA和PMA对映分离的主要驱动力可能是范德华力和氢键相互作用,这些手性化合物的色谱保留是一个焓驱动的过程。分子模拟结果表明,它们对HP-β-CD的手性拆分机制是形成对映异构体与具有不同构象和结合能的HP-β-CD的包合物的原因。HP-β-CD 的结合能与 (S )-异构体大于( R )-异构体,这与( S )-异构体第一次洗脱的实验结果一致。此外,还证实了相互作用能包括范德华能(∆ E vdw)、静电能(∆ E elec)、极性溶剂化能和 SASA 能(∆ E sasa),以及分离因子(α)与旋光异构体和 HP-β-CD 复合物结合能的差异密切相关。同时,由分子动力学模拟可知,Δ( ΔE binding ), (Δ(Δ E binding) = ∆ E binding , R − ∆ E binding , S ) 值的顺序为 MA–HP-β-CD 复合物 > MMA–HP-β-CD 复合物 > PMA–HP-β-CD 复合物,这与从 van't Hoff 图中获得的 Δ(Δ G ) 值的顺序。这表明分子动力学模拟具有手性分辨率的预测功能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号