Molecular and Cellular Endocrinology ( IF 3.8 ) Pub Date : 2021-08-13 , DOI: 10.1016/j.mce.2021.111426 Tiago V Augusto 1 , Cristina Amaral 1 , Cristina F Almeida 1 , Natércia Teixeira 1 , Georgina Correia-da-Silva 1
|
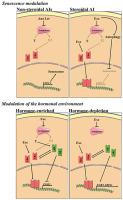
Estrogen receptor-positive (ER+) breast carcinomas are the most common subtype, corresponding to 60% of the cases in premenopausal and 75% in postmenopausal women. The third-generation of aromatase inhibitors (AIs), the non-steroidal Anastrozole (Ana) and Letrozole (Let) and the steroidal Exemestane (Exe), are considered a first-line endocrine therapy for postmenopausal women. Despite their clinical success, the development of resistance is the major setback in clinical practice. Nevertheless, the lack of cross-resistance between AIs hints that these drugs may act through distinct mechanisms. Therefore, this work studied the different effects induced by AIs on biological processes, such as cell proliferation, death, autophagy and senescence. Moreover, their effects on the regulation of the hormonal environment were also explored. The non-steroidal AIs induce senescence, through increased YPEL3 expression, on aromatase-overexpressing breast cancer cells (MCF-7aro), whereas Exe promotes a cytoprotective autophagy, thus blocking senescence induction. In addition, in a hormone-enriched environment, the non-steroidal AIs prevent estrogen signaling, despite up-regulating the estrogen receptor alpha (ERα), while Exe down-regulates ERα and maintains its activation. In these conditions, all AIs up-regulate the androgen receptor (AR) which blocks EGR3 transcription in Exe-treated cells. On the other hand, in hormone-depleted conditions, a crosstalk between AR and ERα occurs, enhancing the estrogenic effects of Exe. This indicates that Exe modulates both ERα and AR, while Ana and Let act as pure AIs. Thus, this study highlights the potential clinical benefit of combining AR antagonists with Exe and discourages the sequential use of Exe as second-line therapy in postmenopausal breast cancer.
中文翻译:

芳香酶抑制剂的不同生物学效应:乳腺癌细胞的凋亡、自噬、衰老和激素状态调节
雌激素受体阳性(ER +) 乳腺癌是最常见的亚型,相当于 60% 的绝经前病例和 75% 的绝经后妇女。第三代芳香化酶抑制剂 (AI) 非甾体阿那曲唑 (Ana) 和来曲唑 (Let) 以及甾体依西美坦 (Exe) 被认为是绝经后妇女的一线内分泌治疗药物。尽管它们在临床上取得了成功,但耐药性的发展是临床实践中的主要挫折。然而,AI 之间缺乏交叉耐药性暗示这些药物可能通过不同的机制发挥作用。因此,这项工作研究了人工智能对生物过程的不同影响,如细胞增殖、死亡、自噬和衰老。此外,还探讨了它们对激素环境调节的影响。非甾体类 AI 诱导衰老,YPEL3在过表达芳香化酶的乳腺癌细胞 (MCF-7aro) 上表达,而 Exe 促进细胞保护性自噬,从而阻断衰老诱导。此外,在激素丰富的环境中,非甾体类 AI 会阻止雌激素信号传导,尽管会上调雌激素受体 α (ERα),而 Exe 会下调 ERα 并维持其活化。在这些情况下,所有 AI 都会上调阻断EGR3的雄激素受体 (AR)Exe 处理的细胞中的转录。另一方面,在激素耗尽的情况下,AR 和 ERα 之间会发生串扰,从而增强 Exe 的雌激素作用。这表明 Exe 调节 ERα 和 AR,而 Ana 和 Let 充当纯 AI。因此,本研究强调了将 AR 拮抗剂与 Exe 联合使用的潜在临床益处,并不鼓励将 Exe 作为绝经后乳腺癌的二线治疗进行序贯使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号