当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of ring size in conformationally restricted ring I analogs of paromomycin on antiribosomal and antibacterial activity
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2021-08-05 , DOI: 10.1039/d1md00214g Michael G Pirrone 1, 2, 3 , Sven N Hobbie 4 , Andrea Vasella 5 , Erik C Böttger 4 , David Crich 1, 2, 6
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2021-08-05 , DOI: 10.1039/d1md00214g Michael G Pirrone 1, 2, 3 , Sven N Hobbie 4 , Andrea Vasella 5 , Erik C Böttger 4 , David Crich 1, 2, 6
Affiliation
|
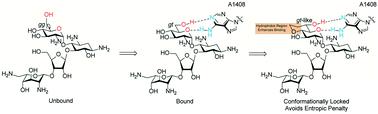
In order to further investigate the importance of the conformation of the ring I side chain in aminoglycoside antibiotic binding to the ribosomal target several derivatives of paromomycin were designed with conformationally locked side chains. By changing the size of the appended ring between O-4′ and C-6′ used to restrict the motion of the side chain, the position of the C-6′ hydroxy group was fine tuned to probe for the optimal conformation for inhibition of the ribosome. While the changes in orientation of the 6′-hydroxy group cannot be completely dissociated from the size and hydrophobicity of the conformation-restricting ring, overall, it is apparent that the preferred conformation of the ring I side chain for interaction with A1408 in the decoding A site of the bacterial ribosome is an ideal gt conformation, which results in the highest antimicrobial activity as well as increased selectivity for bacterial over eukaryotic ribosomes.
中文翻译:

巴龙霉素构象限制环 I 类似物的环大小对抗核糖体和抗菌活性的影响
为了进一步研究环I侧链构象在氨基糖苷类抗生素与核糖体靶标结合中的重要性,设计了几种具有构象锁定侧链的巴龙霉素衍生物。通过改变 O-4' 和 C-6' 之间用于限制侧链运动的附加环的大小,微调 C-6' 羟基的位置,以探索抑制核糖体。虽然 6'-羟基的方向变化不能完全与构象限制环的大小和疏水性分离,但总体而言,很明显,在解码中与 A1408 相互作用的环 I 侧链的优选构象细菌核糖体的一个位点是一个理想的gt构象,这导致最高的抗菌活性以及细菌对真核核糖体的选择性增加。
更新日期:2021-08-13
中文翻译:

巴龙霉素构象限制环 I 类似物的环大小对抗核糖体和抗菌活性的影响
为了进一步研究环I侧链构象在氨基糖苷类抗生素与核糖体靶标结合中的重要性,设计了几种具有构象锁定侧链的巴龙霉素衍生物。通过改变 O-4' 和 C-6' 之间用于限制侧链运动的附加环的大小,微调 C-6' 羟基的位置,以探索抑制核糖体。虽然 6'-羟基的方向变化不能完全与构象限制环的大小和疏水性分离,但总体而言,很明显,在解码中与 A1408 相互作用的环 I 侧链的优选构象细菌核糖体的一个位点是一个理想的gt构象,这导致最高的抗菌活性以及细菌对真核核糖体的选择性增加。



























 京公网安备 11010802027423号
京公网安备 11010802027423号