当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereospecific on-Surface Cyclodehydrogenation of Bishelicenes: Preservation of Handedness from Helical to Planar Chirality
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2021-08-13 , DOI: 10.1002/chem.202102069 Bahaaeddin Irziqat 1 , Aleksandra Cebrat 1 , Miloš Baljozović 1 , Kévin Martin 2 , Manfred Parschau 1 , Narcis Avarvari 2 , Karl-Heinz Ernst 1, 3, 4
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2021-08-13 , DOI: 10.1002/chem.202102069 Bahaaeddin Irziqat 1 , Aleksandra Cebrat 1 , Miloš Baljozović 1 , Kévin Martin 2 , Manfred Parschau 1 , Narcis Avarvari 2 , Karl-Heinz Ernst 1, 3, 4
Affiliation
|
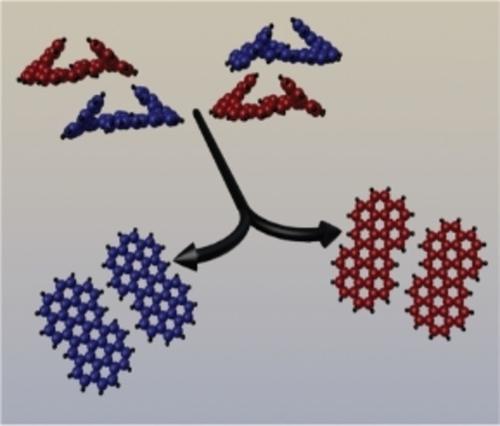
Flattening helices while keeping the handedness: On-surface cyclodehydrogenation of bishelicene enantiomers leads stereospecifically to (M,M) and (P,P) chiral planar polyaromatic hydrocarbons. This is followed by their homochiral aggregation into a 2D conglomerate. Thermally induced cyclodehydrogenation proceeds stereospecifically to chiral, planar coronocoronene. Such a reaction is a special example of topochemistry in which enantiospecific conversion is supported by the alignment of the reactant by the surface.

中文翻译:

Bishelicenes 的立体有择表面环脱氢反应:从螺旋到平面手性的旋向性保存
在保持旋向性的同时压平螺旋:双螺旋烯对映体的表面环化脱氢立体定向地导致(M,M)和(P,P)手性平面聚芳烃。随后是它们的同手性聚集成二维聚合体。热诱导的环脱氢反应立体定向地进行手性、平面冠状晕苯。这种反应是拓扑化学的一个特殊例子,其中对映特异性转化由反应物通过表面的排列来支持。

更新日期:2021-09-24

中文翻译:

Bishelicenes 的立体有择表面环脱氢反应:从螺旋到平面手性的旋向性保存
在保持旋向性的同时压平螺旋:双螺旋烯对映体的表面环化脱氢立体定向地导致(M,M)和(P,P)手性平面聚芳烃。随后是它们的同手性聚集成二维聚合体。热诱导的环脱氢反应立体定向地进行手性、平面冠状晕苯。这种反应是拓扑化学的一个特殊例子,其中对映特异性转化由反应物通过表面的排列来支持。












































 京公网安备 11010802027423号
京公网安备 11010802027423号