当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of a Novel Mesophilic CTP-Dependent Riboflavin Kinase and Rational Engineering to Create Its Thermostable Homologues**
ChemBioChem ( IF 2.6 ) Pub Date : 2021-08-12 , DOI: 10.1002/cbic.202100211 Yashwant Kumar 1 , Reman Kumar Singh 1, 2 , Amrita Brajagopal Hazra 1, 3
ChemBioChem ( IF 2.6 ) Pub Date : 2021-08-12 , DOI: 10.1002/cbic.202100211 Yashwant Kumar 1 , Reman Kumar Singh 1, 2 , Amrita Brajagopal Hazra 1, 3
Affiliation
|
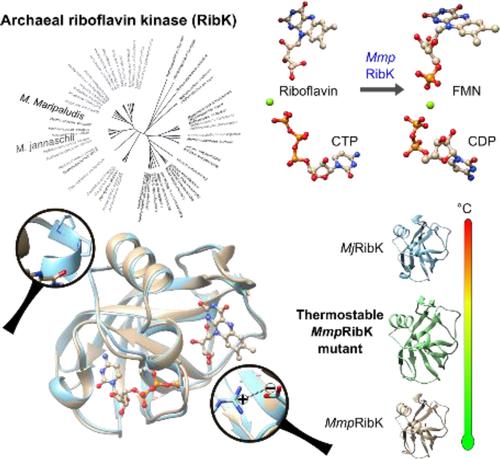
Kinases typically use ATP to phosphorylate their substrates. But not this one! Archaeal riboflavin kinases (RibK), characterized by a unique structural fold, select CTP over all other nucleotides for phosphorylating riboflavin (Vitamin B2) to produce flavin mononucleotide. In this study, we report the characterization of a new mesophilic archaeal RibK from Methanococcus maripaludis. Further, using structural bioinformatics and computational studies, we determined the molecular determinants that confer thermostability to MmpRibK, making this class of enzymes amenable to bioengineering applications.

中文翻译:

一种新型嗜温 CTP 依赖性核黄素激酶的表征和合理工程以创建其耐热同源物**
激酶通常使用 ATP 磷酸化其底物。但不是这个!以独特的结构折叠为特征的古细菌核黄素激酶 (RibK) 选择 CTP 而不是所有其他核苷酸磷酸化核黄素 (维生素 B2) 以产生黄素单核苷酸。在这项研究中,我们报告了来自Methanococcus maripaludis的一种新的嗜温古菌 RibK 的特征。此外,使用结构生物信息学和计算研究,我们确定了赋予 Mmp RibK 热稳定性的分子决定因素,使此类酶适用于生物工程应用。

更新日期:2021-08-12

中文翻译:

一种新型嗜温 CTP 依赖性核黄素激酶的表征和合理工程以创建其耐热同源物**
激酶通常使用 ATP 磷酸化其底物。但不是这个!以独特的结构折叠为特征的古细菌核黄素激酶 (RibK) 选择 CTP 而不是所有其他核苷酸磷酸化核黄素 (维生素 B2) 以产生黄素单核苷酸。在这项研究中,我们报告了来自Methanococcus maripaludis的一种新的嗜温古菌 RibK 的特征。此外,使用结构生物信息学和计算研究,我们确定了赋予 Mmp RibK 热稳定性的分子决定因素,使此类酶适用于生物工程应用。












































 京公网安备 11010802027423号
京公网安备 11010802027423号