当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulating 3d Orbitals of Ni Atoms on Ni-Pt Edge Sites Enables Highly-Efficient Alkaline Hydrogen Evolution
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2021-08-12 , DOI: 10.1002/aenm.202101789 Min Zhou 1 , Hangfei Li 1 , Anchun Long 1 , Bo Zhou 2 , Fei Lu 3 , Fengchu Zhang 3 , Fei Zhan 2 , Zhenxin Zhang 1 , Weiwei Xie 4 , Xianghua Zeng 1 , Ding Yi 3 , Xi Wang 3
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2021-08-12 , DOI: 10.1002/aenm.202101789 Min Zhou 1 , Hangfei Li 1 , Anchun Long 1 , Bo Zhou 2 , Fei Lu 3 , Fengchu Zhang 3 , Fei Zhan 2 , Zhenxin Zhang 1 , Weiwei Xie 4 , Xianghua Zeng 1 , Ding Yi 3 , Xi Wang 3
Affiliation
|
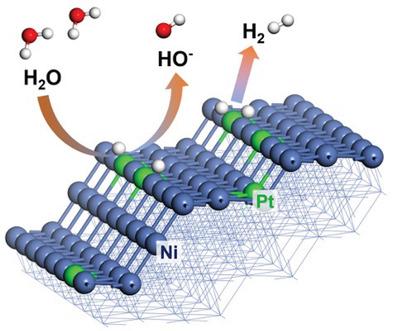
Water electrolysis operating in alkaline environments is a promising route to produce H2 on a massive scale. In this context, designing highly-active and low-cost electrocatalysts is of great importance. Here NiPt alloys with plenty of atomically dispersed Pt at the edges to boost hydrogen evolution in alkaline solution are reported. The formed Ni-Pt atomic pairs at the edges hold engineered electronic structures by reducing the number of coordination atoms to facilitate the kinetically sluggish Volmer step, and further promote the hydrogen coupling step by providing separate active sites as well. With a Pt content of 3at%, this catalyst records an ultralow overpotential of 6 mV to reach the current density of 10 mA cm−2, and delivers a current density of 68.3 mA cm−2 at the overpotential of 30 mV, exceeding that of the commercial 20wt% Pt/C catalyst by a factor of >4. The aberration-corrected transmission electron microscopy and quasi-operando X-ray absorption fine structure measurements show Ni-Pt atomic pairs serve as active sites and enable the subtle adsorption/desorption balances between various intermediates (OH* and H*) during the hydrogen evolution reaction. The as-made alloys show high stability with negligible activity decay after a 12 h chronoamperometric test, addressing its feasibility in an overall water-splitting cell.
中文翻译:

在 Ni-Pt 边缘位点上调节 Ni 原子的 3d 轨道可实现高效的碱性氢演化
在碱性环境中操作的水电解是大规模生产 H 2的有前途的途径。在这种情况下,设计高活性和低成本的电催化剂非常重要。这里报道了在边缘具有大量原子分散 Pt 以促进碱性溶液中析氢的 NiPt 合金。在边缘形成的 Ni-Pt 原子对通过减少配位原子的数量来保持工程电子结构,以促进动力学缓慢的 Volmer 步骤,并通过提供单独的活性位点进一步促进氢偶联步骤。在 Pt 含量为 3 at % 的情况下,该催化剂记录了 6 mV 的超低过电位,以达到 10 mA cm -2的电流密度,并在 30 mV 的过电位下提供 68.3 mA cm -2的电流密度,超过商业 20 wt % Pt/C 催化剂的电流密度> 4。像差校正的透射电子显微镜和准操作数 X 射线吸收精细结构测量显示 Ni-Pt 原子对作为活性位点,并在析氢过程中实现各种中间体(OH* 和 H*)之间的微妙吸附/解吸平衡反应。制成的合金在 12 小时计时电流测试后显示出高稳定性,活性衰减可忽略不计,解决了其在整体水分解电池中的可行性。
更新日期:2021-09-23
中文翻译:

在 Ni-Pt 边缘位点上调节 Ni 原子的 3d 轨道可实现高效的碱性氢演化
在碱性环境中操作的水电解是大规模生产 H 2的有前途的途径。在这种情况下,设计高活性和低成本的电催化剂非常重要。这里报道了在边缘具有大量原子分散 Pt 以促进碱性溶液中析氢的 NiPt 合金。在边缘形成的 Ni-Pt 原子对通过减少配位原子的数量来保持工程电子结构,以促进动力学缓慢的 Volmer 步骤,并通过提供单独的活性位点进一步促进氢偶联步骤。在 Pt 含量为 3 at % 的情况下,该催化剂记录了 6 mV 的超低过电位,以达到 10 mA cm -2的电流密度,并在 30 mV 的过电位下提供 68.3 mA cm -2的电流密度,超过商业 20 wt % Pt/C 催化剂的电流密度> 4。像差校正的透射电子显微镜和准操作数 X 射线吸收精细结构测量显示 Ni-Pt 原子对作为活性位点,并在析氢过程中实现各种中间体(OH* 和 H*)之间的微妙吸附/解吸平衡反应。制成的合金在 12 小时计时电流测试后显示出高稳定性,活性衰减可忽略不计,解决了其在整体水分解电池中的可行性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号