Synlett ( IF 1.7 ) Pub Date : 2021-08-12 , DOI: 10.1055/s-0040-1719824 Juan R. Del Valle , Taylor A. Gerrein , Yassin M. Elbatrawi
|
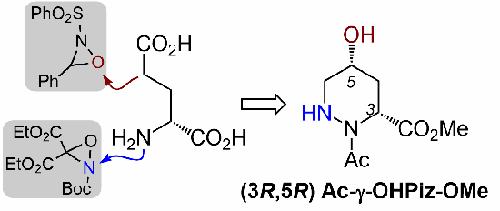
We report an asymmetric synthesis of the (3R,5R)-γ-hydroxypiperazic acid (γ-OHPiz) residue encountered in several bioactive nonribosomal peptides. Our strategy relies on a diastereoselective enolate hydroxylation reaction and electrophilic N-amination to provide the acyclic γ-OHPiz precursor. This orthogonally protected α-hydrazino acid intermediate is amenable to late-stage diazinane ring formation following incorporation into a peptide chain. We determined the N-terminal amide rotamer propensity of the γ-OHPiz residue and showed that the γ-OH substituent enhances trans-amide bias relative to piperazic acid.
中文翻译:

(3R,5R)-γ-羟基哌嗪酸的非对映选择性合成
我们报告了在几种生物活性非核糖体肽中遇到的 (3 R ,5 R )-γ-羟基哌嗪酸 (γ-OHPiz) 残基的不对称合成。我们的策略依赖于非对映选择性烯醇羟基化反应和亲电 N-胺化以提供无环 γ-OHPiz 前体。这种正交保护的 α-肼酸中间体在掺入肽链后适合后期二嗪烷环形成。我们确定了 γ-OHPiz 残基的 N 端酰胺旋转异构体倾向,并表明 γ-OH 取代基增强了相对于哌嗪酸的反式酰胺偏向性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号