Journal of Rare Earths ( IF 5.2 ) Pub Date : 2021-08-13 , DOI: 10.1016/j.jre.2021.08.007 Zhimin Zhao 1 , Jinjia Liu 2, 3 , Gala Sa 1 , Aiju Xu 1
|
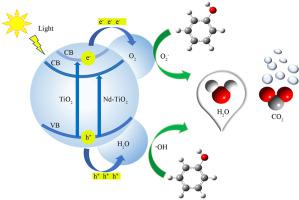
In this study, the photocatalytic activity of Nd-TiO2 photocatalysts obtained by common hydrothermal method was evaluated by practical experiments and theoretical calculations based on density functional theory (DFT). The synthesized photocatalysts were characterized by X-ray diffraction (XRD), N2 adsorption–desorption, Fourier transform infrared spectroscopy (FT-IR), high resolution transmission electron microscopy (HRTEM), X-ray photoelectron spectroscopy (XPS), UV–Vis diffuse reflectance spectroscopy (DRS), and photoluminescence (PL) to study their physical/chemical properties. At the same time, the photoelectronic performance was also investigated. The photodegradation ability of as-prepared photocatalysts and the effect of Nd doped amount and photocatalysts dosage were investigated by the photodegradation of phenol (30 mg/L) under 400 W metal halide lamp (UV–Vis). The effect of Nd on electronic properties of TiO2 and adsorption ability of phenol were discussed. Results show the red-shift wavelength of 0.5 mol%Nd-TiO2, indicating that its absorption capacity is stronger than pristine TiO2 in the same wavelength range. The result of DFT calculations demonstrates that the optical bandgap of Nd-TiO2 is profoundly reduced, thus the light absorption ability is promoted, which will be responsible for the enhanced photocatalytic performance of Nd-TiO2. 0.5 mol% Nd is an optimum value for photodegradation phenol, and phenol can be completely degraded by 0.5 mol%Nd-TiO2 for 210 min, the higher catalytic performance is derived from the efficient separation of e–/h+ pairs. Moreover, the adsorption energy calculations of phenol on TiO2 (101) and Nd-TiO2 (101) demonstrate that the Nd doping can significantly enhance the adsorption ability of phenol on catalyst surfaces because of the formation of Nd–O bonds. At last, the stability measurement through four recycles exhibits that 0.5 mol%Nd-TiO2 possesses excellent stability.
中文翻译:

Nd-TiO2对苯酚的电子特性和光降解能力
本研究基于密度泛函理论(DFT),通过实际实验和理论计算,对常用水热法制备的Nd-TiO 2光催化剂的光催化活性进行了评价。合成的光催化剂通过X射线衍射(XRD)、N 2表征吸附-解吸、傅里叶变换红外光谱 (FT-IR)、高分辨率透射电子显微镜 (HRTEM)、X 射线光电子能谱 (XPS)、紫外-可见漫反射光谱 (DRS) 和光致发光 (PL) 来研究它们物理/化学性质。同时,还研究了光电性能。通过在 400 W 金属卤化物灯 (UV-Vis) 下对苯酚 (30 mg/L) 的光降解研究了所制备的光催化剂的光降解能力以及 Nd 掺杂量和光催化剂用量的影响。讨论了Nd对TiO 2电子性质和苯酚吸附能力的影响。结果显示0.5 mol%Nd-TiO 2的红移波长,表明在相同波长范围内其吸收能力强于原始TiO 2 。DFT计算结果表明,Nd-TiO 2的光学带隙显着减小,从而提高了光吸收能力,这将是Nd-TiO 2光催化性能增强的原因。0.5 mol% Nd是光降解苯酚的最佳值,0.5 mol% Nd-TiO 2 210 min可完全降解苯酚,较高的催化性能来源于e- / h +对的有效分离。此外,苯酚在 TiO 2 (101) 和 Nd-TiO 2上的吸附能计算(101)证明,由于 Nd-O 键的形成,Nd 掺杂可以显着增强苯酚在催化剂表面的吸附能力。最后,通过四次循环的稳定性测量表明,0.5 mol%Nd-TiO 2具有优异的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号