Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2021-08-13 , DOI: 10.1016/j.jmb.2021.167202 Bryan VanSchouwen 1 , Stephen Boulton 2 , Giuseppe Melacini 3
|
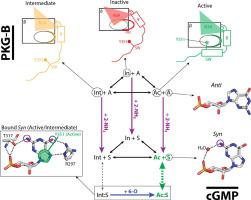
Protein kinase G (PKG) is a major receptor of cGMP, and controls signaling pathways distinct from those regulated by cAMP. However, the contributions of the two substituents that differentiate cGMP from cAMP (i.e. 6-oxo and 2-NH2) to the cGMP-versus-cAMP selectivity of PKG remain unclear. Here, using NMR to map how binding affinity and dynamics of the protein and ligand vary along a ligand double-substitution cycle, we show that the contributions of the two substituents to binding affinity are surprisingly non-additive. Such non-additivity stems primarily from mutual protein–ligand conformational selection, whereby not only does the ligand select for a preferred protein conformation upon binding, but also, the protein selects for a preferred ligand conformation. The 6-oxo substituent mainly controls the conformational equilibrium of the bound protein, while the 2-NH2 substituent primarily controls the conformational equilibrium of the unbound ligand (i.e. syn versus anti). Therefore, understanding the conformational dynamics of both the protein and ligand is essential to explain the cGMP-versus-cAMP selectivity of PKG.
中文翻译:

相互蛋白质配体构象选择驱动蛋白激酶 G 中 cGMP 与 cAMP 的选择性
蛋白激酶 G (PKG) 是 cGMP 的主要受体,控制不同于 cAMP 调节的信号通路。然而,区分 cGMP 和 cAMP 的两个取代基(即6-氧代和 2-NH 2)对 cGMP-与PKG 的 -cAMP 选择性仍不清楚。在这里,使用 NMR 来绘制蛋白质和配体的结合亲和力和动力学如何沿着配体双取代循环变化,我们表明两个取代基对结合亲和力的贡献令人惊讶地是非累加的。这种非可加性主要源于相互的蛋白质-配体构象选择,由此不仅配体在结合时选择优选的蛋白质构象,而且蛋白质也选择优选的配体构象。6-氧代取代基主要控制结合蛋白的构象平衡,而2-NH 2取代基主要控制未结合配体的构象平衡(即syn vs anti)。因此,了解蛋白质和配体的构象动力学对于解释PKG的 cGMP与-cAMP选择性至关重要。










































 京公网安备 11010802027423号
京公网安备 11010802027423号