当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen-Induced Conformational Changes in the PAS-Heme Domain of the Pseudomonas aeruginosa Aer2 Receptor
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-12 , DOI: 10.1021/acs.biochem.1c00452 Emilie Orillard 1 , Selina Anaya 1 , Mark S Johnson 1 , Kylie J Watts 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-12 , DOI: 10.1021/acs.biochem.1c00452 Emilie Orillard 1 , Selina Anaya 1 , Mark S Johnson 1 , Kylie J Watts 1
Affiliation
|
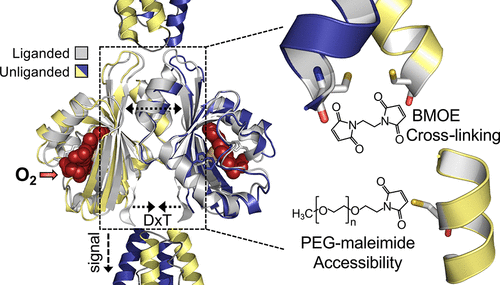
The Aer2 receptor from Pseudomonas aeruginosa has an O2-binding PAS-heme domain that stabilizes O2 via a Trp residue in the distal heme pocket. Trp rotates ∼90° to bond with the ligand and initiate signaling. Although the isolated PAS domain is monomeric, both in solution and in a cyanide-bound crystal structure, an unliganded structure forms a dimer. An overlay of the two structures suggests possible signaling motions but also predicts implausible clashes at the dimer interface when the ligand is bound. Moreover, in a full-length Aer2 dimer, PAS is sandwiched between multiple N- and C-terminal HAMP domains, which would feasibly restrict PAS motions. To explore the PAS dimer interface and signal-induced motions in full-length Aer2, we introduced Cys substitutions and used thiol-reactive probes to examine in vivo accessibility and residue proximities under both aerobic and anaerobic conditions. In vivo, PAS dimers were retained in full-length Aer2 in the presence and absence of O2, and the dimer interface was consistent with the isolated PAS dimer structure. O2-mediated changes were also consistent with structural predictions in which the PAS N-terminal caps move apart and the C-terminal DxT region moves closer together. The DxT motif links PAS to the C-terminal HAMP domains and was critical for PAS-HAMP signaling. Removing the N-terminal HAMP domains altered the distal PAS dimer interface and prevented signaling, even after signal-on lesions were introduced into PAS. The N-terminal HAMP domains thus facilitate the O2-dependent shift of PAS to the signal-on conformation, clarifying their role upstream of the PAS-sensing domain.
中文翻译:

氧诱导的铜绿假单胞菌 Aer2 受体 PAS-血红素结构域的构象变化
来自铜绿假单胞菌的 Aer2 受体具有与 O 2结合的 PAS 血红素结构域,可稳定 O 2通过远端血红素口袋中的色氨酸残基。Trp 旋转 ∼90° 以与配体结合并启动信号传导。尽管分离的 PAS 结构域在溶液和氰化物结合的晶体结构中都是单体,但未配位结构形成二聚体。两种结构的叠加表明可能的信号运动,但也预测了配体结合时二聚体界面上的难以置信的冲突。此外,在全长 Aer2 二聚体中,PAS 夹在多个 N 端和 C 端 HAMP 结构域之间,这可能会限制 PAS 的运动。为了探索全长 Aer2 中的 PAS 二聚体界面和信号诱导的运动,我们引入了 Cys 取代并使用硫醇反应性探针来检查有氧和厌氧条件下的体内可及性和残基接近度。体内,如图2所示,二聚体界面与孤立的PAS二聚体结构一致。O 2介导的变化也与结构预测一致,其中 PAS N 端帽移开而 C 端 DxT 区域更靠近在一起。DxT 基序将 PAS 连接到 C 端 HAMP 结构域,对 PAS-HAMP 信号传导至关重要。去除 N 端 HAMP 结构域会改变远端 PAS 二聚体界面并阻止信号传导,即使在将信号开启病变引入 PAS 之后也是如此。N端HAMP结构域因此促进了PAS依赖于O 2向信号开启构象的转变,阐明了它们在PAS传感结构域上游的作用。
更新日期:2021-08-31
中文翻译:

氧诱导的铜绿假单胞菌 Aer2 受体 PAS-血红素结构域的构象变化
来自铜绿假单胞菌的 Aer2 受体具有与 O 2结合的 PAS 血红素结构域,可稳定 O 2通过远端血红素口袋中的色氨酸残基。Trp 旋转 ∼90° 以与配体结合并启动信号传导。尽管分离的 PAS 结构域在溶液和氰化物结合的晶体结构中都是单体,但未配位结构形成二聚体。两种结构的叠加表明可能的信号运动,但也预测了配体结合时二聚体界面上的难以置信的冲突。此外,在全长 Aer2 二聚体中,PAS 夹在多个 N 端和 C 端 HAMP 结构域之间,这可能会限制 PAS 的运动。为了探索全长 Aer2 中的 PAS 二聚体界面和信号诱导的运动,我们引入了 Cys 取代并使用硫醇反应性探针来检查有氧和厌氧条件下的体内可及性和残基接近度。体内,如图2所示,二聚体界面与孤立的PAS二聚体结构一致。O 2介导的变化也与结构预测一致,其中 PAS N 端帽移开而 C 端 DxT 区域更靠近在一起。DxT 基序将 PAS 连接到 C 端 HAMP 结构域,对 PAS-HAMP 信号传导至关重要。去除 N 端 HAMP 结构域会改变远端 PAS 二聚体界面并阻止信号传导,即使在将信号开启病变引入 PAS 之后也是如此。N端HAMP结构域因此促进了PAS依赖于O 2向信号开启构象的转变,阐明了它们在PAS传感结构域上游的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号