EBioMedicine ( IF 9.7 ) Pub Date : 2021-08-12 , DOI: 10.1016/j.ebiom.2021.103525 Abhijith Biji 1 , Oyahida Khatun 1 , Shachee Swaraj 1 , Rohan Narayan 1 , Raju S Rajmani 2 , Rahila Sardar 3 , Deepshikha Satish 3 , Simran Mehta 4 , Hima Bindhu 4 , Madhumol Jeevan 4 , Deepak K Saini 5 , Amit Singh 1 , Dinesh Gupta 3 , Shashank Tripathi 1
|
Background
While our battle with the COVID-19 pandemic continues, a multitude of Omics data have been generated from patient samples in various studies. Translation of these data into clinical interventions against COVID-19 remains to be accomplished. Exploring host response to COVID-19 in the upper respiratory tract can unveil prognostic markers and therapeutic targets.
Methods
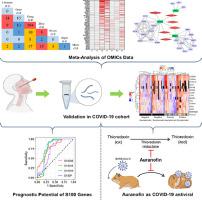
We conducted a meta-analysis of published transcriptome and proteome profiles of respiratory samples of COVID-19 patients to shortlist high confidence upregulated host factors. Subsequently, mRNA overexpression of selected genes was validated in nasal swabs from a cohort of COVID-19 positive/negative, symptomatic/asymptomatic individuals. Guided by this analysis, we sought to check for potential drug targets. An FDA-approved drug, Auranofin, was tested against SARS-CoV-2 replication in cell culture and Syrian hamster challenge model.
Findings
The meta-analysis and validation in the COVID-19 cohort revealed S100 family genes (S100A6, S100A8, S100A9, and S100P) as prognostic markers of severe COVID-19. Furthermore, Thioredoxin (TXN) was found to be consistently upregulated. Auranofin, which targets Thioredoxin reductase, was found to mitigate SARS-CoV-2 replication in vitro. Furthermore, oral administration of Auranofin in Syrian hamsters in therapeutic as well as prophylactic regimen reduced viral replication, IL-6 production, and inflammation in the lungs.
Interpretation
Elevated mRNA level of S100s in the nasal swabs indicate severe COVID-19 disease, and FDA-approved drug Auranofin mitigated SARS-CoV-2 replication in preclinical hamster model.
Funding
This study was supported by the DBT-IISc partnership program (DBT (IED/4/2020-MED/DBT)), the Infosys Young Investigator award (YI/2019/1106), DBT-BIRAC grant (BT/CS0007/CS/02/20) and the DBT-Wellcome Trust India Alliance Intermediate Fellowship (IA/I/18/1/503613) to ST lab.
中文翻译:

通过对鼻咽样本的组学数据进行荟萃分析和验证来识别 COVID-19 预后标志物和治疗靶点
背景
虽然我们与 COVID-19 大流行的斗争仍在继续,但在各种研究中已经从患者样本中生成了大量组学数据。将这些数据转化为针对 COVID-19 的临床干预措施仍有待完成。探索宿主对上呼吸道 COVID-19 的反应可以揭示预后标志物和治疗靶点。
方法
我们对已发表的 COVID-19 患者呼吸道样本的转录组和蛋白质组谱进行了荟萃分析,以筛选出高可信度的上调宿主因子。随后,在来自一组 COVID-19 阳性/阴性、有症状/无症状个体的鼻拭子中验证了所选基因的 mRNA 过表达。在此分析的指导下,我们试图检查潜在的药物靶点。FDA 批准的药物 Auranofin 在细胞培养和叙利亚仓鼠攻击模型中针对 SARS-CoV-2 复制进行了测试。
发现
COVID-19 队列中的荟萃分析和验证显示 S100 家族基因(S100A6、S100A8、S100A9 和 S100P)是严重 COVID-19 的预后标志物。此外,发现硫氧还蛋白 (TXN) 持续上调。发现以硫氧还蛋白还原酶为靶点的金诺芬在体外可以减轻 SARS-CoV-2 的复制。此外,在治疗和预防方案中,在叙利亚仓鼠中口服金诺芬可减少病毒复制、IL-6 产生和肺部炎症。
解释
鼻拭子中 S100s 的 mRNA 水平升高表明严重的 COVID-19 疾病,FDA 批准的药物金诺芬减轻了临床前仓鼠模型中的 SARS-CoV-2 复制。
资金
本研究得到 DBT-IISc 合作计划 (DBT (IED/4/2020-MED/DBT))、Infosys 青年研究者奖 (YI/2019/1106)、DBT-BIRAC 赠款 (BT/CS0007/CS/ 02/20) 和 DBT-Wellcome Trust India Alliance Intermediate Fellowship (IA/I/18/1/503613) 到 ST 实验室。










































 京公网安备 11010802027423号
京公网安备 11010802027423号