当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Cascade Carbonylation to α,β-Unsaturated Piperidones via Selective Cleavage of Carbon–Carbon Triple Bonds
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-08-12 , DOI: 10.1002/anie.202108120 Yao Ge 1 , Fei Ye 1, 2 , Ji Yang 1 , Anke Spannenberg 1 , Haijun Jiao 1 , Ralf Jackstell 1 , Matthias Beller 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-08-12 , DOI: 10.1002/anie.202108120 Yao Ge 1 , Fei Ye 1, 2 , Ji Yang 1 , Anke Spannenberg 1 , Haijun Jiao 1 , Ralf Jackstell 1 , Matthias Beller 1
Affiliation
|
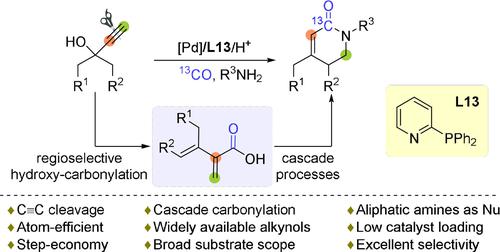
A direct and selective synthesis of α,β-unsaturated piperidones by a new palladium-catalyzed cascade carbonylation is described. In the presented protocol, easily available propargylic alcohols react with aliphatic amines to provide a broad variety of interesting heterocycles. Key to the success of this transformation is a remarkable catalytic cleavage of the present carbon–carbon triple bond by using a specific catalyst with 2-diphenylphosphinopyridine as ligand and appropriate reaction conditions. Mechanistic studies and control experiments revealed branched unsaturated acid 11 as crucial intermediate.
中文翻译:

钯催化通过选择性裂解碳-碳三键进行级联羰基化生成 α,β-不饱和哌啶酮
描述了通过新型钯催化级联羰基化直接选择性合成 α,β-不饱和哌啶酮。在所提出的方案中,容易获得的炔丙醇与脂肪胺反应,提供多种有趣的杂环。这种转化成功的关键是通过使用以 2-二苯基膦吡啶为配体的特定催化剂和适当的反应条件,对现有的碳-碳三键进行显着的催化裂解。机理研究和控制实验表明支链不饱和酸11是关键的中间体。
更新日期:2021-09-27
中文翻译:

钯催化通过选择性裂解碳-碳三键进行级联羰基化生成 α,β-不饱和哌啶酮
描述了通过新型钯催化级联羰基化直接选择性合成 α,β-不饱和哌啶酮。在所提出的方案中,容易获得的炔丙醇与脂肪胺反应,提供多种有趣的杂环。这种转化成功的关键是通过使用以 2-二苯基膦吡啶为配体的特定催化剂和适当的反应条件,对现有的碳-碳三键进行显着的催化裂解。机理研究和控制实验表明支链不饱和酸11是关键的中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号