当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bifacial PNAs Destabilize MALAT1 by 3′ A-Tail Displacement from the U-Rich Internal Loop
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-08-12 , DOI: 10.1021/acschembio.1c00575 Shiqin Miao 1 , Debmalya Bhunia 1 , Shekaraiah Devari 1 , Yufeng Liang 1 , Oliver Munyaradzi 1 , Sarah Rundell 1 , Dennis Bong 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-08-12 , DOI: 10.1021/acschembio.1c00575 Shiqin Miao 1 , Debmalya Bhunia 1 , Shekaraiah Devari 1 , Yufeng Liang 1 , Oliver Munyaradzi 1 , Sarah Rundell 1 , Dennis Bong 1
Affiliation
|
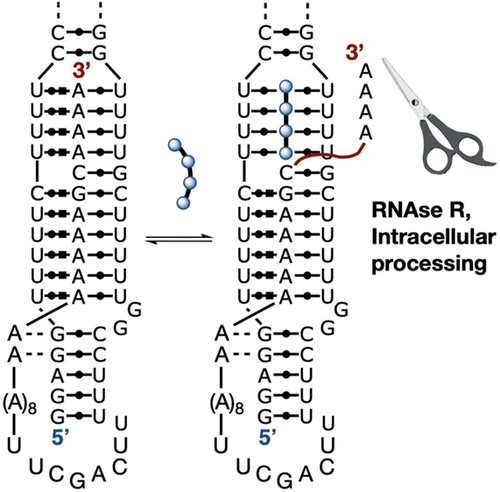
We report herein a new class of synthetic reagents for targeting the element for nuclear expression (ENE) in MALAT1, a long noncoding RNA upregulated in many cancers. The cis-acting ENE contains a U-rich internal loop (URIL) that forms an 11 base UAU-rich triplex stem with the truncated 3′ oligo-A tail of MALAT1, protecting the terminus from exonuclease digestion and greatly extending transcript lifetime. Bifacial peptide nucleic acids (bPNAs) similarly bind URILs via base triple formation between two uracil bases and a synthetic base, melamine. We synthesized a set of low molecular weight bPNAs composed of α-linked peptide, isodipeptide, and diketopiperazine backbones and evaluated their ENE binding efficacy in vitrovia oligo-A strand displacement and consequent exonuclease sensitivity. Degradation was greatly enhanced by bPNA treatment in the presence of exonucleases, with ENE half-life plunging to 6 min from >24 h. RNA digestion kinetics could clearly distinguish between bPNAs with similar URIL affinities, highlighting the utility of functional assays for evaluating synthetic RNA binders. In vitro activity was mirrored by a 50% knockdown of MALAT1 expression in pancreatic cancer (PANC-1) cells upon treatment with bPNAs, consistent with intracellular digestion triggered by a similar ENE A-tail displacement mechanism. Pulldown from PANC-1 total RNA with biotinylated bPNA enriched MALAT1 > 4000× , supportive of bPNA-URIL selectivity. Together, these experiments establish the feasibility of native transcript targeting by bPNA in both in vitro and intracellular contexts. Reagents such as bPNAs may be useful tools for the investigation of transcripts stabilized by cis-acting poly(A) binding RNA elements.
中文翻译:

双面 PNA 通过来自富 U 型内环的 3' A 尾位移使 MALAT1 不稳定
我们在此报告了一类新的合成试剂,用于靶向 MALAT1 中的核表达元件 (ENE),MALAT1 是一种在许多癌症中上调的长链非编码 RNA。所述顺式作用ENE含有富含U的内部循环(脲),其形成一个11个碱基富UAU-三缸与MALAT1的截短的3'寡A尾干,保护从外切核酸酶消化的末端,并且大大延长转录寿命。双面肽核酸 (bPNA) 类似地通过两个尿嘧啶碱基和合成碱基三聚氰胺之间的碱基三重形成结合 URIL 。我们合成了一组由 α 连接肽、异二肽和二酮哌嗪骨架组成的低分子量 bPNA,并通过以下方法评估了它们在体外的 ENE 结合功效oligo-A 链置换和随之而来的核酸外切酶敏感性。在核酸外切酶存在的情况下,bPNA 处理大大增强了降解,ENE 半衰期从 >24 小时降至 6 分钟。RNA 消化动力学可以清楚地区分具有相似 URIL 亲和力的 bPNA,突出了功能测定在评估合成 RNA 结合剂方面的效用。体外用 bPNA 处理后,胰腺癌 (PANC-1) 细胞中 MALAT1 表达的 50% 敲低反映了活性,这与由类似的 ENE A 尾置换机制触发的细胞内消化一致。从 PANC-1 总 RNA 中提取的生物素化 bPNA 富集 MALAT1 > 4000×,支持 bPNA-URIL 选择性。总之,这些实验确定了 bPNA在体外和细胞内环境中靶向天然转录物的可行性。bPNA 等试剂可能是研究由顺式作用 poly(A) 结合 RNA 元件稳定的转录物的有用工具。
更新日期:2021-08-20
中文翻译:

双面 PNA 通过来自富 U 型内环的 3' A 尾位移使 MALAT1 不稳定
我们在此报告了一类新的合成试剂,用于靶向 MALAT1 中的核表达元件 (ENE),MALAT1 是一种在许多癌症中上调的长链非编码 RNA。所述顺式作用ENE含有富含U的内部循环(脲),其形成一个11个碱基富UAU-三缸与MALAT1的截短的3'寡A尾干,保护从外切核酸酶消化的末端,并且大大延长转录寿命。双面肽核酸 (bPNA) 类似地通过两个尿嘧啶碱基和合成碱基三聚氰胺之间的碱基三重形成结合 URIL 。我们合成了一组由 α 连接肽、异二肽和二酮哌嗪骨架组成的低分子量 bPNA,并通过以下方法评估了它们在体外的 ENE 结合功效oligo-A 链置换和随之而来的核酸外切酶敏感性。在核酸外切酶存在的情况下,bPNA 处理大大增强了降解,ENE 半衰期从 >24 小时降至 6 分钟。RNA 消化动力学可以清楚地区分具有相似 URIL 亲和力的 bPNA,突出了功能测定在评估合成 RNA 结合剂方面的效用。体外用 bPNA 处理后,胰腺癌 (PANC-1) 细胞中 MALAT1 表达的 50% 敲低反映了活性,这与由类似的 ENE A 尾置换机制触发的细胞内消化一致。从 PANC-1 总 RNA 中提取的生物素化 bPNA 富集 MALAT1 > 4000×,支持 bPNA-URIL 选择性。总之,这些实验确定了 bPNA在体外和细胞内环境中靶向天然转录物的可行性。bPNA 等试剂可能是研究由顺式作用 poly(A) 结合 RNA 元件稳定的转录物的有用工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号