Cancer Cell ( IF 48.8 ) Pub Date : 2021-08-12 , DOI: 10.1016/j.ccell.2021.07.005 Qing Zhou 1 , Chong-Rui Xu 1 , Ying Cheng 2 , Yun-Peng Liu 3 , Gong-Yan Chen 4 , Jiu-Wei Cui 5 , Nong Yang 6 , Yong Song 7 , Xiao-Ling Li 8 , Shun Lu 9 , Jian-Ying Zhou 10 , Zhi-Yong Ma 11 , Shi-Ying Yu 12 , Cheng Huang 13 , Yong-Qian Shu 14 , Zhen Wang 1 , Jin-Ji Yang 1 , Hai-Yan Tu 1 , Wen-Zhao Zhong 1 , Yi-Long Wu 1
|
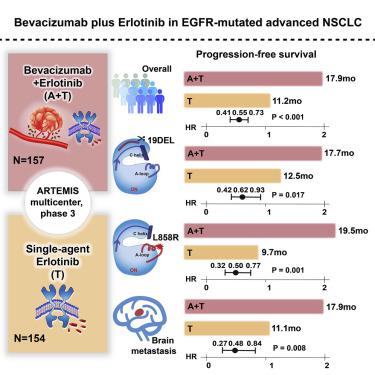
Dual inhibition of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) pathways may delay therapeutic resistance in advanced non-small cell lung cancer (NSCLC). This phase 3 study investigated the efficacy and safety of an erlotinib plus bevacizumab regimen in untreated patients with advanced NSCLC. In total, 311 patients received bevacizumab plus erlotinib (n = 157) or erlotinib only (n = 154). Progression-free survival (PFS) was 17.9 months (95% confidence interval [CI], 15.2–19.9) for bevacizumab plus erlotinib and 11.2 months (95% CI, 9.7–13.8) for erlotinib only (hazard ratio [HR] = 0.55; 95% CI, 0.41–0.73; p < 0.001). A brain metastases subgroup treated with bevacizumab plus erlotinib also showed improved PFS (HR = 0.48; 95% CI, 0.27–0.84; p = 0.008). Grade ≥3 treatment-related adverse events occurred in 86 (54.8%) and 40 (26.1%) patients, respectively. Bevacizumab plus erlotinib significantly improved PFS in patients with untreated metastatic EGFR-mutated NSCLC, including those with brain metastases at baseline.











































 京公网安备 11010802027423号
京公网安备 11010802027423号