Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic Glycomacromolecules of Defined Valency, Absolute Configuration, and Topology Distinguish between Human Lectins
JACS Au ( IF 8.5 ) Pub Date : 2021-08-10 , DOI: 10.1021/jacsau.1c00255 Manuel Hartweg 1 , Yivan Jiang 1 , Gokhan Yilmaz 2, 3 , Cassie M Jarvis 1 , Hung V-T Nguyen 1 , Gastón A Primo 4 , Alessandra Monaco 3 , Valentin P Beyer 3 , Kathleen K Chen 1 , Somesh Mohapatra 5 , Simon Axelrod 5 , Rafael Gómez-Bombarelli 5 , Laura L Kiessling 1 , C Remzi Becer 3, 4 , Jeremiah A Johnson 1
JACS Au ( IF 8.5 ) Pub Date : 2021-08-10 , DOI: 10.1021/jacsau.1c00255 Manuel Hartweg 1 , Yivan Jiang 1 , Gokhan Yilmaz 2, 3 , Cassie M Jarvis 1 , Hung V-T Nguyen 1 , Gastón A Primo 4 , Alessandra Monaco 3 , Valentin P Beyer 3 , Kathleen K Chen 1 , Somesh Mohapatra 5 , Simon Axelrod 5 , Rafael Gómez-Bombarelli 5 , Laura L Kiessling 1 , C Remzi Becer 3, 4 , Jeremiah A Johnson 1
Affiliation
|
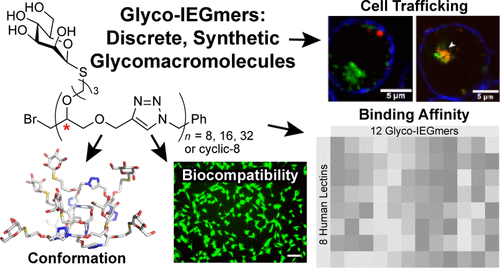
Carbohydrate-binding proteins (lectins) play vital roles in cell recognition and signaling, including pathogen binding and innate immunity. Thus, targeting lectins, especially those on the surface of immune cells, could advance immunology and drug discovery. Lectins are typically oligomeric; therefore, many of the most potent ligands are multivalent. An effective strategy for lectin targeting is to display multiple copies of a single glycan epitope on a polymer backbone; however, a drawback to such multivalent ligands is they cannot distinguish between lectins that share monosaccharide binding selectivity (e.g., mannose-binding lectins) as they often lack molecular precision. Here, we describe the development of an iterative exponential growth (IEG) synthetic strategy that enables facile access to synthetic glycomacromolecules with precisely defined and tunable sizes up to 22.5 kDa, compositions, topologies, and absolute configurations. Twelve discrete mannosylated “glyco-IEGmers” are synthesized and screened for binding to a panel of mannoside-binding immune lectins (DC-SIGN, DC-SIGNR, MBL, SP-D, langerin, dectin-2, mincle, and DEC-205). In many cases, the glyco-IEGmers had distinct length, stereochemistry, and topology-dependent lectin-binding preferences. To understand these differences, we used molecular dynamics and density functional theory simulations of octameric glyco-IEGmers, which revealed dramatic effects of glyco-IEGmer stereochemistry and topology on solution structure and reveal an interplay between conformational diversity and chiral recognition in selective lectin binding. Ligand function also could be controlled by chemical substitution: by tuning the side chains of glyco-IEGmers that bind DC-SIGN, we could alter their cellular trafficking through alteration of their aggregation state. These results highlight the power of precision synthetic oligomer/polymer synthesis for selective biological targeting, motivating the development of next-generation glycomacromolecules tailored for specific immunological or other therapeutic applications.
中文翻译:

具有确定化合价、绝对构型和拓扑结构的合成糖大分子可区分人类凝集素
碳水化合物结合蛋白(凝集素)在细胞识别和信号传导中发挥着重要作用,包括病原体结合和先天免疫。因此,针对凝集素,尤其是免疫细胞表面的凝集素,可以促进免疫学和药物发现。凝集素通常是寡聚的;因此,许多最有效的配体是多价的。凝集素靶向的有效策略是在聚合物主链上展示单个聚糖表位的多个副本;然而,这种多价配体的缺点是它们无法区分具有单糖结合选择性的凝集素(例如甘露糖结合凝集素),因为它们通常缺乏分子精度。在这里,我们描述了迭代指数增长(IEG)合成策略的开发,该策略能够轻松获得具有精确定义和可调尺寸(高达 22.5 kDa)、组成、拓扑和绝对构型的合成糖大分子。合成并筛选了 12 个离散的甘露糖基化“糖基 IEGmers”,用于与一组甘露糖苷结合免疫凝集素(DC-SIGN、DC-SIGNR、MBL、SP-D、langerin、dectin-2、mincle 和 DEC-205)结合)。在许多情况下,糖-IEGmer 具有不同的长度、立体化学和拓扑依赖性凝集素结合偏好。为了理解这些差异,我们使用八聚糖-IEGmer的分子动力学和密度泛函理论模拟,揭示了糖-IEGmer立体化学和拓扑对溶液结构的巨大影响,并揭示了选择性凝集素结合中构象多样性和手性识别之间的相互作用。 配体功能也可以通过化学取代来控制:通过调整结合DC-SIGN的糖-IEGmer的侧链,我们可以通过改变它们的聚集状态来改变它们的细胞运输。这些结果凸显了精确合成低聚物/聚合物合成用于选择性生物靶向的能力,推动了针对特定免疫学或其他治疗应用定制的下一代糖大分子的开发。
更新日期:2021-08-10
中文翻译:

具有确定化合价、绝对构型和拓扑结构的合成糖大分子可区分人类凝集素
碳水化合物结合蛋白(凝集素)在细胞识别和信号传导中发挥着重要作用,包括病原体结合和先天免疫。因此,针对凝集素,尤其是免疫细胞表面的凝集素,可以促进免疫学和药物发现。凝集素通常是寡聚的;因此,许多最有效的配体是多价的。凝集素靶向的有效策略是在聚合物主链上展示单个聚糖表位的多个副本;然而,这种多价配体的缺点是它们无法区分具有单糖结合选择性的凝集素(例如甘露糖结合凝集素),因为它们通常缺乏分子精度。在这里,我们描述了迭代指数增长(IEG)合成策略的开发,该策略能够轻松获得具有精确定义和可调尺寸(高达 22.5 kDa)、组成、拓扑和绝对构型的合成糖大分子。合成并筛选了 12 个离散的甘露糖基化“糖基 IEGmers”,用于与一组甘露糖苷结合免疫凝集素(DC-SIGN、DC-SIGNR、MBL、SP-D、langerin、dectin-2、mincle 和 DEC-205)结合)。在许多情况下,糖-IEGmer 具有不同的长度、立体化学和拓扑依赖性凝集素结合偏好。为了理解这些差异,我们使用八聚糖-IEGmer的分子动力学和密度泛函理论模拟,揭示了糖-IEGmer立体化学和拓扑对溶液结构的巨大影响,并揭示了选择性凝集素结合中构象多样性和手性识别之间的相互作用。 配体功能也可以通过化学取代来控制:通过调整结合DC-SIGN的糖-IEGmer的侧链,我们可以通过改变它们的聚集状态来改变它们的细胞运输。这些结果凸显了精确合成低聚物/聚合物合成用于选择性生物靶向的能力,推动了针对特定免疫学或其他治疗应用定制的下一代糖大分子的开发。










































 京公网安备 11010802027423号
京公网安备 11010802027423号