当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Importance of High-Valent Iron Complex and Reactive Radicals in Organic Contaminants’ Abatement by the Fe-TAML/Free Chlorine System
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-08-11 , DOI: 10.1021/acsestengg.1c00124 Binbin Shao 1 , Jiahui Zhu 1 , Gongming Zhou 1 , Bingcai Pan 2 , Xin Zhang 3 , Lei Dong 1, 3 , Xiaohong Guan 1, 4
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-08-11 , DOI: 10.1021/acsestengg.1c00124 Binbin Shao 1 , Jiahui Zhu 1 , Gongming Zhou 1 , Bingcai Pan 2 , Xin Zhang 3 , Lei Dong 1, 3 , Xiaohong Guan 1, 4
Affiliation
|
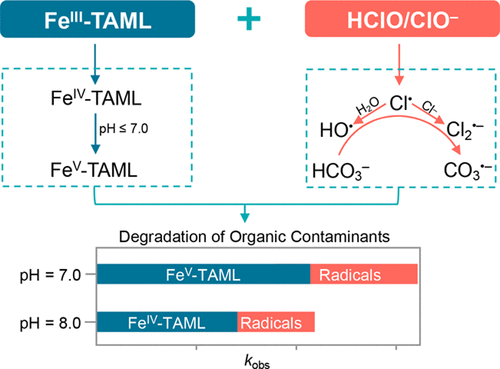
This study demonstrated the formation of high-valent iron complex as well as free radicals in the reaction of iron(III)-tetraamidomacrocyclic ligand (FeIII-TAML) with free chlorine. FeIV(O)-TAML was generated at pH 5.0–10.0, while FeIV(O)-TAML can be further transformed into FeV(O)-TAML by free chlorine at pH 5.0–7.0. The one-electron reduction of free chlorine formed Cl•. The performance of the FeIII-TAML/free chlorine system toward organic contaminants was evaluated by investigating the degradation of three target organic contaminants with different functional groups. The pseudo-first-order rate constant (kobs) of organic contaminants’ degradation by the FeIII-TAML/free chlorine system was about 400–2000-fold greater than that by the FeIII-TAML/hydrogen peroxide (H2O2) system at pH 7.0. FeV(O)-TAML and FeIV(O)-TAML were proposed as the major high-valent iron oxidants responsible for organic contaminants’ degradation at pH 5.0–7.0 and at pH 8.0–10.0, respectively. Additionally, Cl• and secondary free radicals (e.g., Cl2•– and HO•) were responsible for organic contaminants’ degradation. The FeIII-TAML/free chlorine system might be a promising oxidation process because both selective oxidants (high-valent iron complex) and nonselective oxidants (free radicals) contribute to decomposition of organic contaminants in this process.
中文翻译:

高价铁络合物和活性自由基在 Fe-TAML/游离氯系统去除有机污染物中的重要性
该研究证明了在铁 (III)-四酰胺大环配体 (Fe III -TAML) 与游离氯的反应中形成了高价铁络合物和自由基。Fe IV (O)-TAML 在 pH 5.0-10.0 时生成,而 Fe IV (O)-TAML在 pH 5.0-7.0 时可通过游离氯进一步转化为 Fe V (O)-TAML。游离氯的单电子还原形成Cl •。通过研究具有不同官能团的三种目标有机污染物的降解情况,评估了 Fe III -TAML/游离氯系统对有机污染物的性能。伪一阶速率常数 ( k obs)在 pH 7.0 条件下,Fe III -TAML/游离氯系统对有机污染物的降解率比 Fe III -TAML/过氧化氢 (H 2 O 2 ) 系统的降解率高约 400-2000 倍。Fe V (O)-TAML 和 Fe IV (O)-TAML 被认为是主要的高价铁氧化剂,分别负责在 pH 5.0-7.0 和 pH 8.0-10.0 下有机污染物的降解。此外,Cl •和二级自由基(例如,Cl 2 •–和HO •)是造成有机污染物降解的原因。Fe III-TAML/游离氯系统可能是一种很有前景的氧化过程,因为选择性氧化剂(高价铁络合物)和非选择性氧化剂(自由基)都有助于在该过程中分解有机污染物。
更新日期:2021-10-08
中文翻译:

高价铁络合物和活性自由基在 Fe-TAML/游离氯系统去除有机污染物中的重要性
该研究证明了在铁 (III)-四酰胺大环配体 (Fe III -TAML) 与游离氯的反应中形成了高价铁络合物和自由基。Fe IV (O)-TAML 在 pH 5.0-10.0 时生成,而 Fe IV (O)-TAML在 pH 5.0-7.0 时可通过游离氯进一步转化为 Fe V (O)-TAML。游离氯的单电子还原形成Cl •。通过研究具有不同官能团的三种目标有机污染物的降解情况,评估了 Fe III -TAML/游离氯系统对有机污染物的性能。伪一阶速率常数 ( k obs)在 pH 7.0 条件下,Fe III -TAML/游离氯系统对有机污染物的降解率比 Fe III -TAML/过氧化氢 (H 2 O 2 ) 系统的降解率高约 400-2000 倍。Fe V (O)-TAML 和 Fe IV (O)-TAML 被认为是主要的高价铁氧化剂,分别负责在 pH 5.0-7.0 和 pH 8.0-10.0 下有机污染物的降解。此外,Cl •和二级自由基(例如,Cl 2 •–和HO •)是造成有机污染物降解的原因。Fe III-TAML/游离氯系统可能是一种很有前景的氧化过程,因为选择性氧化剂(高价铁络合物)和非选择性氧化剂(自由基)都有助于在该过程中分解有机污染物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号