当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Drug–Polymer Interactions in Acetaminophen/Hydroxypropylmethylcellulose Acetyl Succinate Amorphous Solid Dispersions Revealed by Multidimensional Multinuclear Solid-State NMR Spectroscopy
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-08-10 , DOI: 10.1021/acs.molpharmaceut.1c00427 Andrea Pugliese 1 , Michael Toresco 2 , Daniel McNamara 3 , Dinu Iuga 4 , Anuji Abraham 3 , Michael Tobyn 5 , Lucy E Hawarden 5 , Frédéric Blanc 1, 6
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-08-10 , DOI: 10.1021/acs.molpharmaceut.1c00427 Andrea Pugliese 1 , Michael Toresco 2 , Daniel McNamara 3 , Dinu Iuga 4 , Anuji Abraham 3 , Michael Tobyn 5 , Lucy E Hawarden 5 , Frédéric Blanc 1, 6
Affiliation
|
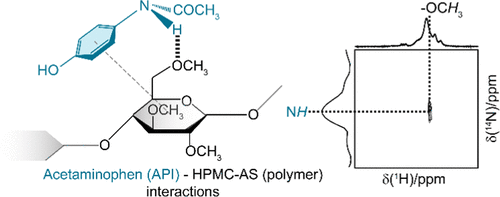
The bioavailability of insoluble crystalline active pharmaceutical ingredients (APIs) can be enhanced by formulation as amorphous solid dispersions (ASDs). One of the key factors of ASD stabilization is the formation of drug–polymer interactions at the molecular level. Here, we used a range of multidimensional and multinuclear nuclear magnetic resonance (NMR) experiments to identify these interactions in amorphous acetaminophen (paracetamol)/hydroxypropylmethylcellulose acetyl succinate (HPMC-AS) ASDs at various drug loadings. At low drug loading (<20 wt %), we showed that 1H–13C through-space heteronuclear correlation experiments identify proximity between aromatic protons in acetaminophen with cellulose backbone protons in HPMC-AS. We also show that 14N–1H heteronuclear multiple quantum coherence (HMQC) experiments are a powerful approach in probing spatial interactions in amorphous materials and establish the presence of hydrogen bonds (H-bond) between the amide nitrogen of acetaminophen with the cellulose ring methyl protons in these ASDs. In contrast, at higher drug loading (40 wt %), no acetaminophen/HPMC-AS spatial proximity was identified and domains of recrystallization of amorphous acetaminophen into its crystalline form I, the most thermodynamically stable polymorph, and form II are identified. These results provide atomic scale understanding of the interactions in the acetaminophen/HPMC-AS ASD occurring via H-bond interactions.
中文翻译:

多维多核固态核磁共振波谱揭示对乙酰氨基酚/羟丙基甲基纤维素乙酰琥珀酸酯无定形固体分散体中的药物-聚合物相互作用
不溶性结晶活性药物成分(API)的生物利用度可以通过配制为无定形固体分散体(ASD)来提高。 ASD 稳定的关键因素之一是在分子水平上形成药物-聚合物相互作用。在这里,我们使用了一系列多维和多核核磁共振 (NMR) 实验来识别不同载药量的无定形对乙酰氨基酚(扑热息痛)/羟丙基甲基纤维素乙酰琥珀酸酯 (HPMC-AS) ASD 中的这些相互作用。在低药物负载量下(<20 id=38>1 H– 13 C 穿越空间异核相关实验确定了对乙酰氨基酚中的芳香质子与 HPMC-AS 中的纤维素骨架质子之间的接近性。我们还表明14 N– 1 H 异核多量子相干性 (HMQC) 实验是探测无定形材料中空间相互作用的有效方法,并确定对乙酰氨基酚的酰胺氮与这些 ASD 中的纤维素环甲基质子之间是否存在氢键(H 键)。负载量(40 wt%),没有发现对乙酰氨基酚/HPMC-AS 空间邻近性,并且确定了无定形对乙酰氨基酚重结晶为其晶型 I(热力学最稳定的多晶型物)和晶型 II 的区域。这些结果提供了对乙酰氨基酚的原子尺度理解。对乙酰氨基酚/HPMC-AS ASD 中的相互作用通过氢键相互作用发生。
更新日期:2021-09-06
中文翻译:

多维多核固态核磁共振波谱揭示对乙酰氨基酚/羟丙基甲基纤维素乙酰琥珀酸酯无定形固体分散体中的药物-聚合物相互作用
不溶性结晶活性药物成分(API)的生物利用度可以通过配制为无定形固体分散体(ASD)来提高。 ASD 稳定的关键因素之一是在分子水平上形成药物-聚合物相互作用。在这里,我们使用了一系列多维和多核核磁共振 (NMR) 实验来识别不同载药量的无定形对乙酰氨基酚(扑热息痛)/羟丙基甲基纤维素乙酰琥珀酸酯 (HPMC-AS) ASD 中的这些相互作用。在低药物负载量下(<20 id=38>1 H– 13 C 穿越空间异核相关实验确定了对乙酰氨基酚中的芳香质子与 HPMC-AS 中的纤维素骨架质子之间的接近性。我们还表明14 N– 1 H 异核多量子相干性 (HMQC) 实验是探测无定形材料中空间相互作用的有效方法,并确定对乙酰氨基酚的酰胺氮与这些 ASD 中的纤维素环甲基质子之间是否存在氢键(H 键)。负载量(40 wt%),没有发现对乙酰氨基酚/HPMC-AS 空间邻近性,并且确定了无定形对乙酰氨基酚重结晶为其晶型 I(热力学最稳定的多晶型物)和晶型 II 的区域。这些结果提供了对乙酰氨基酚的原子尺度理解。对乙酰氨基酚/HPMC-AS ASD 中的相互作用通过氢键相互作用发生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号