Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-08-11 , DOI: 10.1016/j.jmgm.2021.108005 Shelley H Burnaman 1 , Daniel W Kneller 2 , Yuan-Fang Wang 1 , Andrey Kovalevsky 2 , Irene T Weber 1
|
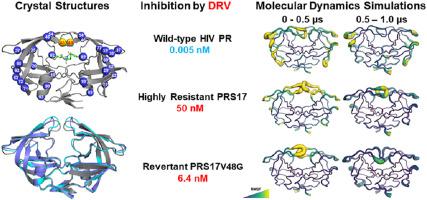
Drug resistance is a serious problem for controlling the HIV/AIDS pandemic. Current antiviral drugs show several orders of magnitude worse inhibition of highly resistant clinical variant PRS17 of HIV-1 protease compared with wild-type protease. We have analyzed the effects of a common resistance mutation G48V in the flexible flaps of the protease by assessing the revertant PRS17V48G for changes in enzyme kinetics, inhibition, structure, and dynamics. Both PRS17 and the revertant showed about 10-fold poorer catalytic efficiency than wild-type enzyme (0.55 and 0.39 μM−1min−1 compared to 6.3 μM−1min−1). Clinical inhibitors, amprenavir and darunavir, showed 2-fold and 8-fold better inhibition, respectively, of the revertant than of PRS17, although the inhibition constants for PRS17V48G were still 25 to 1,200-fold worse than for wild-type protease. Crystal structures of inhibitor-free revertant and amprenavir complexes with revertant and PRS17 were solved at 1.3–1.5 Å resolution. The amprenavir complexes of PRS17V48G and PRS17 showed no significant differences in the interactions with inhibitor, although changes were observed in the conformation of Phe53 and the interactions of the flaps. The inhibitor-free structure of the revertant showed flaps in an open conformation, however, the flap tips do not have the unusual curled conformation seen in inhibitor-free PRS17. Molecular dynamics simulations were run for 1 μs on the two inhibitor-free mutants and wild-type protease. PRS17 exhibited higher conformational fluctuations than the revertant, while the wild-type protease adopted the closed conformation and showed the least variation. The second half of the simulations captured the transition of the flaps of PRS17 from a closed to a semi-open state, whereas the flaps of PRS17V48G tucked into the active site and the wild-type protease retained the closed conformation. These results suggest that mutation G48V contributes to drug resistance by altering the conformational dynamics of the flaps.
中文翻译:

回复突变 V48G 改变高度耐药 HIV 蛋白酶 PRS17 的构象动力学
耐药性是控制艾滋病毒/艾滋病流行的一个严重问题。与野生型蛋白酶相比,目前的抗病毒药物对 HIV-1 蛋白酶的高度耐药临床变异 PRS17 的抑制效果要差几个数量级。我们通过评估回复型 PRS17 V48G在酶动力学、抑制、结构和动力学方面的变化,分析了蛋白酶柔性瓣中常见的抗性突变 G48V 的影响。PRS17 和回复体均显示出比野生型酶低约 10 倍的催化效率(0.55 和 0.39 μM -1 min -1相比 6.3 μM -1 min -1)。尽管 PRS17 V48G的抑制常数仍比野生型蛋白酶低 25 至 1,200 倍,但临床抑制剂安普那韦和地瑞那韦对回复体的抑制作用分别是 PRS17 的 2 倍和 8 倍。以 1.3–1.5 Å 分辨率解析无抑制剂回复体和安普那韦复合体与回复体和 PRS17 的晶体结构。PRS17 V48G的安普那韦复合物和 PRS17 在与抑制剂的相互作用方面没有显着差异,尽管在 Phe53 的构象和皮瓣的相互作用中观察到了变化。回复体的无抑制剂结构显示皮瓣呈开放构象,然而,皮瓣尖端没有在不含抑制剂的 PRS17 中看到的不寻常的卷曲构象。对两种不含抑制剂的突变体和野生型蛋白酶进行 1 μs 的分子动力学模拟。PRS17的构象波动高于回复体,而野生型蛋白酶采用闭合构象,变异最小。模拟的后半部分捕捉到 PRS17 的襟翼从关闭状态到半打开状态的过渡,而 PRS17 V48G的襟翼塞入活性位点,野生型蛋白酶保持闭合构象。这些结果表明突变 G48V 通过改变皮瓣的构象动力学有助于耐药性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号