Cell Host & Microbe ( IF 20.6 ) Pub Date : 2021-08-11 , DOI: 10.1016/j.chom.2021.07.006 Charles Bou-Nader 1 , Frauke Muecksch 2 , Janae B Brown 3 , Jackson M Gordon 1 , Ashley York 2 , Chen Peng 2 , Rodolfo Ghirlando 1 , Michael F Summers 4 , Paul D Bieniasz 5 , Jinwei Zhang 1
|
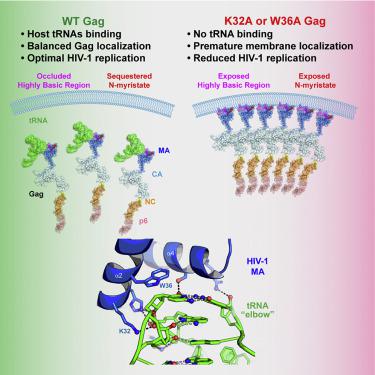
The HIV-1 virion structural polyprotein, Gag, is directed to particle assembly sites at the plasma membrane by its N-terminal matrix (MA) domain. MA also binds to host tRNAs. To understand the molecular basis of MA-tRNA interaction and its potential function, we present a co-crystal structure of HIV-1 MA-tRNALys3 complex. The structure reveals a specialized group of MA basic and aromatic residues preconfigured to recognize the distinctive structure of the tRNA elbow. Mutational, cross-linking, fluorescence, and NMR analyses show that the crystallographically defined interface drives MA-tRNA binding in solution and living cells. The structure indicates that MA is unlikely to bind tRNA and membrane simultaneously. Accordingly, single-amino-acid substitutions that abolish MA-tRNA binding caused striking redistribution of Gag to the plasma membrane and reduced HIV-1 replication. Thus, HIV-1 exploits host tRNAs to occlude a membrane localization signal and control the subcellular distribution of its major structural protein.
中文翻译:

HIV-1基质-tRNA复合物结构揭示了宿主控制Gag定位的基础
HIV-1 病毒体结构多蛋白 Gag 通过其 N 端基质 (MA) 结构域引导至质膜上的颗粒组装位点。 MA 还与宿主 tRNA 结合。为了了解 MA-tRNA 相互作用的分子基础及其潜在功能,我们提出了 HIV-1 MA-tRNA Lys3复合物的共晶结构。该结构揭示了一组专门的 MA 碱性残基和芳香残基,这些残基预先配置用于识别 tRNA 肘部的独特结构。突变、交联、荧光和 NMR 分析表明,晶体学定义的界面驱动 MA-tRNA 在溶液和活细胞中结合。该结构表明 MA 不太可能同时结合 tRNA 和膜。因此,消除 MA-tRNA 结合的单氨基酸取代导致 Gag 显着重新分布到质膜并减少 HIV-1 复制。因此,HIV-1利用宿主tRNA来封闭膜定位信号并控制其主要结构蛋白的亚细胞分布。











































 京公网安备 11010802027423号
京公网安备 11010802027423号