当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Lewis Basic Amine Site on Proton Reduction Activity of NNN-Co Pincer Complex
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2021-08-11 , DOI: 10.1002/bkcs.12373 Seungjin Song 1 , Jaewhan Cho 1 , Hyeonjeong Jo 1 , Junseong Lee 2 , Jun‐Ho Choi 1 , Junhyeok Seo 1
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2021-08-11 , DOI: 10.1002/bkcs.12373 Seungjin Song 1 , Jaewhan Cho 1 , Hyeonjeong Jo 1 , Junseong Lee 2 , Jun‐Ho Choi 1 , Junhyeok Seo 1
Affiliation
|
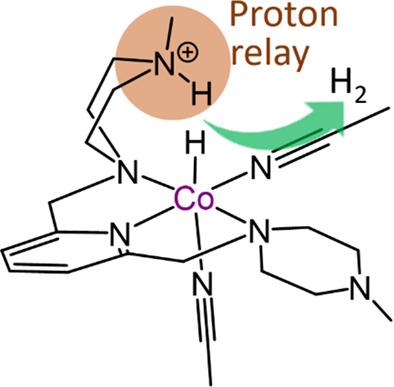
Electrochemical proton reduction is a promising energy storage method because H2 molecule has a simple structure with a relatively low potential energy. Current interest in hydrogen catalysts has increased research efforts on synthetic analogs of hydrogenase active sites. In this study, we demonstrated the electrochemical H2 evolution reactivity of [NNNR-Co(CH3CN)3]2+ (R CH2 (1b), NCH3 (2b)) complexes and examined a proton-relay process in the H2 evolution reaction (HER). Upon one-electron reduction, the Co(II) ion center in a high-spin state dissociated a CH3CN ligand, while opening a reaction site. Cyclic voltammograms of the Co complexes indicated quasi-reversible Co(II/I) redox behaviors, and both complexes 1b and 2b showed catalytic H2 evolution activity. Interestingly, 2b, assisted by a proton-relaying NCH3 group, exhibited more efficient catalytic activity than 1b.
中文翻译:

Lewis碱式胺位对NNN-Co钳形配合物质子还原活性的影响
电化学质子还原是一种很有前景的储能方法,因为H 2分子结构简单,势能相对较低。目前对氢催化剂的兴趣增加了对氢化酶活性位点合成类似物的研究工作。在本研究中,我们展示了[NNN R -Co(CH 3 CN) 3 ] 2+ (R CH 2 ( 1b ), NCH 3 ( 2b )) 配合物的电化学 H 2析出反应性,并检查了质子中继过程在 H 2进化反应(HER)。在单电子还原时,处于高自旋状态的 Co(II) 离子中心解离了 CH 3 CN 配体,同时打开了一个反应位点。Co 配合物的循环伏安图表明 Co(II/I) 具有准可逆的氧化还原行为,配合物1b和2b均显示出催化 H 2析出活性。有趣的是,在质子中继 NCH 3基团的辅助下,2b表现出比1b更有效的催化活性。
更新日期:2021-08-11
中文翻译:

Lewis碱式胺位对NNN-Co钳形配合物质子还原活性的影响
电化学质子还原是一种很有前景的储能方法,因为H 2分子结构简单,势能相对较低。目前对氢催化剂的兴趣增加了对氢化酶活性位点合成类似物的研究工作。在本研究中,我们展示了[NNN R -Co(CH 3 CN) 3 ] 2+ (R CH 2 ( 1b ), NCH 3 ( 2b )) 配合物的电化学 H 2析出反应性,并检查了质子中继过程在 H 2进化反应(HER)。在单电子还原时,处于高自旋状态的 Co(II) 离子中心解离了 CH 3 CN 配体,同时打开了一个反应位点。Co 配合物的循环伏安图表明 Co(II/I) 具有准可逆的氧化还原行为,配合物1b和2b均显示出催化 H 2析出活性。有趣的是,在质子中继 NCH 3基团的辅助下,2b表现出比1b更有效的催化活性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号