Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergistic Catalysis by Single-Atom Catalysts and Redox Mediator to Improve Lithium–Oxygen Batteries Performance
Small ( IF 13.0 ) Pub Date : 2021-08-10 , DOI: 10.1002/smll.202101620 Danying Li 1 , Kangli Xu 1 , Maogen Zhu 1 , Tao Xu 1 , Zhechen Fan 1 , Linqin Zhu 1 , Yongchun Zhu 1
Small ( IF 13.0 ) Pub Date : 2021-08-10 , DOI: 10.1002/smll.202101620 Danying Li 1 , Kangli Xu 1 , Maogen Zhu 1 , Tao Xu 1 , Zhechen Fan 1 , Linqin Zhu 1 , Yongchun Zhu 1
Affiliation
|
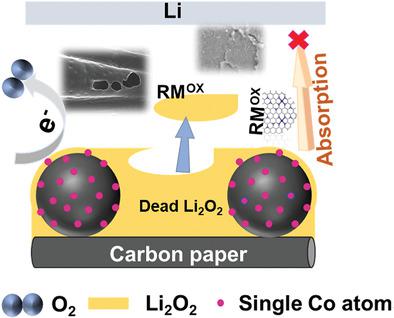
Lithium–oxygen (Li–O2) batteries with ultrahigh theoretical energy density have attracted widespread attention while there are still problems with high overpotential and poor cycle stability. Rational design and application of efficient catalysts to improve the performance of Li–O2 batteries is of significant importance. In this work, Co single atoms catalysts are successfully combined with redox mediator (lithium bromide [LiBr]) to synergistically catalyze electrochemical reactions in Li–O2 batteries. Single-atom cobalt anchored in porous N-doped hollow carbon spheres (CoSAs-NHCS) with high specific surface area and high catalytic activity are utilized as cathode material. However, the potential performances of batteries are difficult to adequately achieve with only CoSAs-NHCS, owing to the blocked electrochemical active sites covered by insulating solid-state discharge product Li2O2. Combined with LiBr as redox mediator, the enhanced OER catalytic effect extends throughout all formed Li2O2 during discharge. Meantime, the certain adsorption effect of CoSAs-NHCS on Br2 and Br3− can reduce the shuttle of RMox. The synergistic effect of Co single atoms and LiBr can not only promote more Li2O2 decomposition but also reduce the shuttle effect by absorbing the oxidized redox mediator. Li–O2 batteries with Co single atoms and LiBr achieve ultralow overpotential of 0.69 V and longtime stable cyclability.
中文翻译:

单原子催化剂和氧化还原介体协同催化提高锂氧电池性能
具有超高理论能量密度的锂氧(Li-O 2)电池受到广泛关注,但仍存在过电位高和循环稳定性差的问题。合理设计和应用高效催化剂对提高Li-O 2电池的性能具有重要意义。在这项工作中,Co 单原子催化剂成功地与氧化还原介质(溴化锂 [LiBr])结合以协同催化 Li-O 2 中的电化学反应电池。将单原子钴锚定在具有高比表面积和高催化活性的多孔 N 掺杂空心碳球 (CoSAs-NHCS) 中作为正极材料。然而,由于绝缘固态放电产物 Li 2 O 2覆盖了封闭的电化学活性位点,因此仅使用 CoSAs-NHCS 难以充分实现电池的潜在性能。与作为氧化还原介质的 LiBr 相结合,增强的 OER 催化效果在放电过程中延伸到所有形成的 Li 2 O 2中。同时,CoSAs-NHCS对Br 2和Br 3 −的一定吸附作用可以减少RM ox的穿梭. Co单原子和LiBr的协同作用不仅可以促进更多的Li 2 O 2分解,还可以通过吸收氧化的氧化还原介质来减少穿梭效应。具有 Co 单原子和 LiBr 的Li-O 2电池实现了 0.69 V 的超低过电位和长期稳定的循环性能。
更新日期:2021-09-23
中文翻译:

单原子催化剂和氧化还原介体协同催化提高锂氧电池性能
具有超高理论能量密度的锂氧(Li-O 2)电池受到广泛关注,但仍存在过电位高和循环稳定性差的问题。合理设计和应用高效催化剂对提高Li-O 2电池的性能具有重要意义。在这项工作中,Co 单原子催化剂成功地与氧化还原介质(溴化锂 [LiBr])结合以协同催化 Li-O 2 中的电化学反应电池。将单原子钴锚定在具有高比表面积和高催化活性的多孔 N 掺杂空心碳球 (CoSAs-NHCS) 中作为正极材料。然而,由于绝缘固态放电产物 Li 2 O 2覆盖了封闭的电化学活性位点,因此仅使用 CoSAs-NHCS 难以充分实现电池的潜在性能。与作为氧化还原介质的 LiBr 相结合,增强的 OER 催化效果在放电过程中延伸到所有形成的 Li 2 O 2中。同时,CoSAs-NHCS对Br 2和Br 3 −的一定吸附作用可以减少RM ox的穿梭. Co单原子和LiBr的协同作用不仅可以促进更多的Li 2 O 2分解,还可以通过吸收氧化的氧化还原介质来减少穿梭效应。具有 Co 单原子和 LiBr 的Li-O 2电池实现了 0.69 V 的超低过电位和长期稳定的循环性能。










































 京公网安备 11010802027423号
京公网安备 11010802027423号