Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-08-10 , DOI: 10.1016/j.jmgm.2021.108006 Mehdi D Esrafili 1 , Parisasadat Mousavian 2
|
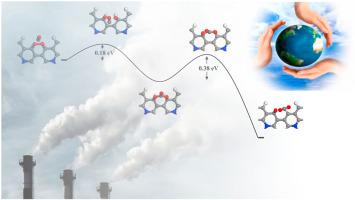
Density functional theory calculations, including dispersion effects, are used to demonstrate how substitutional nitrogen atoms can improve the catalytic reactivity of graphene nanoribbons (GNR) with edge defects in the CO oxidation process. It is demonstrated that the addition of nitrogen impurities significantly enhances O2 adsorption on GNR. Carbon atoms near the edges of defects are the most active sites for capturing O2 molecules. The lower adsorption energy of CO relative to O2 implies that the N-modified GNR is resistant to CO poisoning. The Eley-Rideal (E-R) mechanism has activation energies as low as 0.38 eV, making it the most energetically relevant pathway for the CO + O2 reaction. The findings of this study might help in the design of catalysts for metal-free catalysis of CO oxidation.
中文翻译:

具有边缘缺陷的 N 取代石墨烯纳米带上的催化 CO 氧化反应
密度泛函理论计算,包括色散效应,用于证明置换氮原子如何提高具有 CO 氧化过程中边缘缺陷的石墨烯纳米带 (GNR) 的催化反应性。结果表明,氮杂质的加入显着增强了 GNR 上的O 2吸附。缺陷边缘附近的碳原子是捕获O 2分子的最活跃的位置。CO 相对于 O 2的较低吸附能意味着 N 改性的 GNR 具有抗 CO 中毒的能力。Eley-Rideal (ER) 机制的活化能低至 0.38 eV,使其成为 CO + O 2与能量最相关的途径反应。这项研究的结果可能有助于设计用于无金属催化 CO 氧化的催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号