当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Semirational engineering of an aldo–keto reductase KmAKR for overcoming trade-offs between catalytic activity and thermostability
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2021-08-10 , DOI: 10.1002/bit.27913 Shu-Fang Li 1, 2, 3 , Jian-Yong Xie 1, 2, 3 , Shuai Qiu 1, 2, 3 , Shen-Yuan Xu 1, 2, 3 , Feng Cheng 1, 2, 3 , Ya-Jun Wang 1, 2, 3 , Yu-Guo Zheng 1, 2, 3
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2021-08-10 , DOI: 10.1002/bit.27913 Shu-Fang Li 1, 2, 3 , Jian-Yong Xie 1, 2, 3 , Shuai Qiu 1, 2, 3 , Shen-Yuan Xu 1, 2, 3 , Feng Cheng 1, 2, 3 , Ya-Jun Wang 1, 2, 3 , Yu-Guo Zheng 1, 2, 3
Affiliation
|
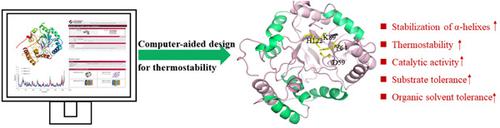
Enzyme engineering usually generates trade-offs between activity, stability, and selectivity. Herein, we report semirational engineering of an aldo–keto reductase (AKR) KmAKR for simultaneously enhancing its thermostability and catalytic activity. Previously, we constructed KmAKRM9 (W297H/Y296W/K29H/Y28A/T63M/A30P/T302S/N109K/S196C), which showed outstanding activity towards t-butyl 6-chloro-(3R,5S)-dihydroxyhexanoate ((3R,5S)-CDHH), and t-butyl 6-cyano-(3R,5R)-dihydroxyhexanoate, the key chiral building blocks of rosuvastatin and atorvastatin. Under the guidance of computer-aided design including consensus residues analysis and molecular dynamics (MD) simulations, K164, S182, S232, and Q266 were dug out for their thermostability conferring roles, generating the “best” mutant KmAKRM13(W297H/Y296W/K29H/Y28A/T63M/A30P/T302S/N109K/S196C/K164E/S232A/S182H/Q266D). The Tm and T5015 values of KmAKRM13 were 10.4 and 6.1°C higher than that of KmAKRM9, respectively. Moreover, it displayed a significantly elevated organic solvent tolerance over KmAKRM9. Structural analysis indicated that stabilization of the α-helixes mainly contributed to thermostability enhancement. Under the optimized conditions, KmAKRM13 completely asymmetrically reduced 400 g/l t-butyl 6-chloro-(5S)-hydroxy-3-oxohexanoate ((5S)-CHOH) in 8.0 h at a high substrate to catalyst ratio (S/C) of 106.7 g/g, giving diastereomerically pure (3R,5S)-CDHH (>99.5% d.e.P) with a space-time yield (STY) of 449.2 g/l·d.
中文翻译:

醛酮还原酶 KmAKR 的半理性工程,用于克服催化活性和热稳定性之间的权衡
酶工程通常会在活性、稳定性和选择性之间进行权衡。在此,我们报告了醛酮还原酶 (AKR) Km AKR 的半理性工程,以同时增强其热稳定性和催化活性。此前,我们构建了Km AKR M9 (W297H/Y296W/K29H/Y28A/T63M/A30P/T302S/N109K/S196C),对6-氯-(3 R ,5 S )-二羟基己酸叔丁酯(( 3 R ,5 S )-CDHH) 和叔丁基 6-氰基-(3 R ,5 R)-二羟基己酸酯,罗苏伐他汀和阿托伐他汀的关键手性构件。在包括共有残基分析和分子动力学 (MD) 模拟在内的计算机辅助设计的指导下,K164、S182、S232 和 Q266 因其赋予热稳定性的作用而被挖掘出来,产生了“最佳”突变体Km AKR M13 (W297H/Y296W /K29H/Y28A/T63M/A30P/T302S/N109K/S196C/K164E/S232A/S182H/Q266D)。Km AKR M13的T m和T 50 15值分别比Km AKR M9高10.4 和6.1°C 。此外,它显示出显着提高的有机溶剂耐受性公里AKR M9。结构分析表明,α-螺旋的稳定主要有助于提高热稳定性。在优化的条件下,Km AKR M13在高底物催化剂比下,在 8.0 小时内完全不对称还原了 400 g/l 6-氯-(5 S )-羟基-3-氧代己酸叔丁酯 ((5 S )-CHOH) (S/C) 为 106.7 g/g,得到非对映异构体纯 (3 R ,5 S )-CDHH (>99.5% de . P ),时空产率 (STY) 为 449.2 g/l·d。
更新日期:2021-10-13
中文翻译:

醛酮还原酶 KmAKR 的半理性工程,用于克服催化活性和热稳定性之间的权衡
酶工程通常会在活性、稳定性和选择性之间进行权衡。在此,我们报告了醛酮还原酶 (AKR) Km AKR 的半理性工程,以同时增强其热稳定性和催化活性。此前,我们构建了Km AKR M9 (W297H/Y296W/K29H/Y28A/T63M/A30P/T302S/N109K/S196C),对6-氯-(3 R ,5 S )-二羟基己酸叔丁酯(( 3 R ,5 S )-CDHH) 和叔丁基 6-氰基-(3 R ,5 R)-二羟基己酸酯,罗苏伐他汀和阿托伐他汀的关键手性构件。在包括共有残基分析和分子动力学 (MD) 模拟在内的计算机辅助设计的指导下,K164、S182、S232 和 Q266 因其赋予热稳定性的作用而被挖掘出来,产生了“最佳”突变体Km AKR M13 (W297H/Y296W /K29H/Y28A/T63M/A30P/T302S/N109K/S196C/K164E/S232A/S182H/Q266D)。Km AKR M13的T m和T 50 15值分别比Km AKR M9高10.4 和6.1°C 。此外,它显示出显着提高的有机溶剂耐受性公里AKR M9。结构分析表明,α-螺旋的稳定主要有助于提高热稳定性。在优化的条件下,Km AKR M13在高底物催化剂比下,在 8.0 小时内完全不对称还原了 400 g/l 6-氯-(5 S )-羟基-3-氧代己酸叔丁酯 ((5 S )-CHOH) (S/C) 为 106.7 g/g,得到非对映异构体纯 (3 R ,5 S )-CDHH (>99.5% de . P ),时空产率 (STY) 为 449.2 g/l·d。











































 京公网安备 11010802027423号
京公网安备 11010802027423号