当前位置:
X-MOL 学术
›
Isr. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
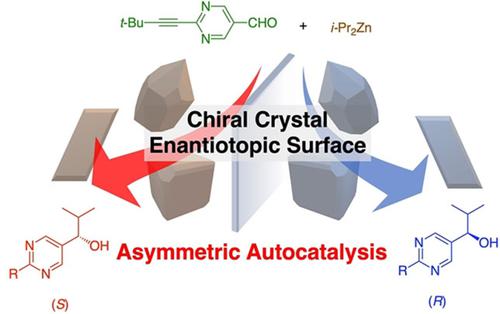
Asymmetric Autocatalysis as a Link Between Crystal Chirality and Highly Enantioenriched Organic Compounds
Israel Journal of Chemistry ( IF 3.2 ) Pub Date : 2021-08-09 , DOI: 10.1002/ijch.202100047 Kenso Soai 1, 2 , Arimasa Matsumoto 3 , Tsuneomi Kawasaki 1
Israel Journal of Chemistry ( IF 3.2 ) Pub Date : 2021-08-09 , DOI: 10.1002/ijch.202100047 Kenso Soai 1, 2 , Arimasa Matsumoto 3 , Tsuneomi Kawasaki 1
Affiliation
|
The origin of biological homochirality has attracted much attention. Pyrimidyl alkanols exhibit asymmetric autocatalysis with significant amplification of enantiomeric excess in the reaction between pyrimidine-5-carbaldehyde and diisopropyl zinc (the Soai reaction). Chiral inorganic crystals such as quartz, cinnabar and sodium chlorate act as chiral triggers of asymmetric autocatalysis to afford highly enantioenriched pyrimidyl alkanol, with the absolute configurations corresponding to those of the chiral crystals. Chiral organic crystals composed of achiral organic compounds such as cytosine, adenine dinitrate, and γ-glycine act as the chiral triggers of the Soai reaction to afford pyrimidyl alkanol with high ee that corresponds to the absolute configuration of the chiral trigger. This review describes that chiral minerals and chiral organic crystals act as the chiral triggers of the Soai reaction and that these chiral crystals are correlated to near-enantiopure chiral organic compounds.
中文翻译:

不对称自催化作为晶体手性和高度对映体富集的有机化合物之间的联系
生物同手性的起源备受关注。嘧啶基链烷醇表现出不对称自催化,在嘧啶-5-甲醛和二异丙基锌之间的反应(Soai 反应)中对映体过量显着增加。手性无机晶体如石英、朱砂和氯酸钠可作为不对称自催化的手性触发剂,得到高度对映体富集的嘧啶烷醇,其绝对构型与手性晶体的构型相对应。由非手性有机化合物(如胞嘧啶、二硝酸腺嘌呤和 γ-甘氨酸)组成的手性有机晶体作为 Soai 反应的手性触发器,提供具有高 ee 的嘧啶基烷醇,与手性触发器的绝对配置相对应。
更新日期:2021-08-09
中文翻译:

不对称自催化作为晶体手性和高度对映体富集的有机化合物之间的联系
生物同手性的起源备受关注。嘧啶基链烷醇表现出不对称自催化,在嘧啶-5-甲醛和二异丙基锌之间的反应(Soai 反应)中对映体过量显着增加。手性无机晶体如石英、朱砂和氯酸钠可作为不对称自催化的手性触发剂,得到高度对映体富集的嘧啶烷醇,其绝对构型与手性晶体的构型相对应。由非手性有机化合物(如胞嘧啶、二硝酸腺嘌呤和 γ-甘氨酸)组成的手性有机晶体作为 Soai 反应的手性触发器,提供具有高 ee 的嘧啶基烷醇,与手性触发器的绝对配置相对应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号