Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-08-09 , DOI: 10.1016/j.jmgm.2021.108002 Shemphang Hynniewta 1 , Makroni Lily 2 , Asit K Chandra 1
|
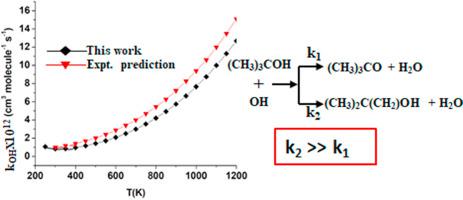
The kinetics of the gas-phase atmospheric reaction of t-butanol with OH radicals is computationally studied using the CCSD(T)/aug-cc-pVTZ//M06-2X/6–311++G(d,p) level of calculation. The rate coefficients are evaluated for a wide temperature range of 250–1200 K and the calculated rate coefficient value of at is in close agreement with experimental results. The H-abstraction from the –CH3 group is predicted to be the main reaction channel. A weak negative temperature dependence of rate coefficient is observed in 250–300 K. The study also highlighted the possibility of re-generation of OH radicals at higher temperature. The ozone formation potential of t-butanol in the troposphere has also been estimated and discussed.
中文翻译:

叔丁醇与OH自由基反应动力学及臭氧形成势的计算研究
使用 CCSD(T)/aug-cc-pVTZ//M06-2X/6–311++G(d,p) 水平计算研究叔丁醇与 OH 自由基的气相大气反应动力学计算。速率系数在 250-1200 K 的宽温度范围内进行评估,计算的速率系数值为 在 与实验结果非常吻合。来自–CH 3基团的H-抽象被预测为主要反应通道。在 250-300 K 中观察到速率系数的弱负温度依赖性。该研究还强调了在更高温度下再生 OH 自由基的可能性。还对对流层中叔丁醇的臭氧形成潜力进行了估计和讨论。











































 京公网安备 11010802027423号
京公网安备 11010802027423号