当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergetic effect of C and Ni on hydrogen release from Mg–Ni-electrochemically synthesized reduced graphene oxide based hydride
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2021-08-02 , DOI: 10.1039/d1se00842k Srikanta Panda 1, 2, 3, 4 , Marla V. V. Satya Aditya 1, 2, 3, 4 , Sankara Sarma V. Tatiparti 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2021-08-02 , DOI: 10.1039/d1se00842k Srikanta Panda 1, 2, 3, 4 , Marla V. V. Satya Aditya 1, 2, 3, 4 , Sankara Sarma V. Tatiparti 1, 2, 3, 4
Affiliation
|
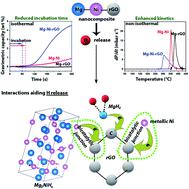
Mg–reduced graphene oxide (rGO), Mg–Ni and Mg–Ni–rGO nanocomposites were synthesized by ball milling. They were subsequently hydrogenated at PH2 ≈ 15 bar, ∼320 °C. Isothermal (∼320 °C) and non-isothermal (room temperature – 450 °C, 5 °C min−1) H release experiments were performed on the hydrogenated nanocomposites. The incubation time (dH/dt|320°C ≈ 0) during isothermal H release drops from ∼15 min in Mg–rGO to ∼75 s in Mg–Ni and ∼60 s in Mg–Ni–rGO. The onset temperature during non-isothermal H release reduces from ∼350 °C in Mg–rGO to ∼275 °C in Mg–Ni–rGO. The reasons for the superior H release by Mg–Ni–rGO are investigated. Ball milling of Mg–rGO develops persistent Mg–C interaction at ∼283 eV from C1s X-ray photoelectron spectroscopy (XPS), involving electron transfer between Mg and C. In Mg–Ni, a non-stoichiometric Mg2NiHx phase and MgH2 unit cell shrinkage develop upon H uptake resulting in improved H release. Mg–Ni–rGO possesses unreacted metallic Ni (X-ray diffraction) upon ball milling; Ni–C interaction (∼283.5–284.5 eV, C1s XPS) and red shift in G band (Raman) vis-à-vis Mg–rGO upon H uptake. Interestingly, Mg–Ni–rGO exhibits the individual effects of rGO and Ni addition. The Ni–C interaction from metallic Ni and possibly from Mg2NiHx lead to the superior H release by Mg–Ni–rGO. This work demonstrates the synergetic effect of adding multiple catalysts on H release.
中文翻译:

C和Ni对Mg-Ni电化学合成还原氧化石墨烯基氢化物释放氢的协同作用
通过球磨法合成了镁还原氧化石墨烯(rGO)、Mg-Ni 和 Mg-Ni-rGO 纳米复合材料。它们随后在P H2 ≈ 15 bar、∼320 °C 下氢化。对氢化纳米复合材料进行等温(~320°C)和非等温(室温 – 450°C,5°C min -1)H 释放实验。孵育时间 (dH/d t | 320°C≈ 0) 在等温 H 释放期间从 Mg-rGO 中的~15 分钟下降到 Mg-Ni 中的~75 秒和 Mg-Ni-rGO 中的~60 秒。非等温 H 释放期间的起始温度从 Mg-rGO 中的~350°C 降低到 Mg-Ni-rGO 中的~275°C。研究了 Mg-Ni-rGO 释放 H 的优越性的原因。从 C1s X 射线光电子能谱 (XPS) 中,Mg-rGO 的球磨产生了约 283 eV 的持久 Mg-C 相互作用,涉及 Mg 和 C 之间的电子转移。在 Mg-Ni 中,非化学计量的 Mg 2 NiH x相和MgH 2晶胞收缩随着H吸收而发展,导致H释放得到改善。Mg-Ni-rGO 在球磨时具有未反应的金属 Ni(X 射线衍射);Ni-C 相互作用(~283.5-284.5 eV,C1s XPS)和 G 带(拉曼)相对于Mg-rGO 对 H 的吸收。有趣的是,Mg-Ni-rGO 表现出 rGO 和 Ni 添加的单独效应。来自金属 Ni 和可能来自 Mg 2 NiH x的 Ni-C 相互作用导致 Mg-Ni-rGO 优异的 H 释放。这项工作证明了添加多种催化剂对 H 释放的协同作用。
更新日期:2021-08-09
中文翻译:

C和Ni对Mg-Ni电化学合成还原氧化石墨烯基氢化物释放氢的协同作用
通过球磨法合成了镁还原氧化石墨烯(rGO)、Mg-Ni 和 Mg-Ni-rGO 纳米复合材料。它们随后在P H2 ≈ 15 bar、∼320 °C 下氢化。对氢化纳米复合材料进行等温(~320°C)和非等温(室温 – 450°C,5°C min -1)H 释放实验。孵育时间 (dH/d t | 320°C≈ 0) 在等温 H 释放期间从 Mg-rGO 中的~15 分钟下降到 Mg-Ni 中的~75 秒和 Mg-Ni-rGO 中的~60 秒。非等温 H 释放期间的起始温度从 Mg-rGO 中的~350°C 降低到 Mg-Ni-rGO 中的~275°C。研究了 Mg-Ni-rGO 释放 H 的优越性的原因。从 C1s X 射线光电子能谱 (XPS) 中,Mg-rGO 的球磨产生了约 283 eV 的持久 Mg-C 相互作用,涉及 Mg 和 C 之间的电子转移。在 Mg-Ni 中,非化学计量的 Mg 2 NiH x相和MgH 2晶胞收缩随着H吸收而发展,导致H释放得到改善。Mg-Ni-rGO 在球磨时具有未反应的金属 Ni(X 射线衍射);Ni-C 相互作用(~283.5-284.5 eV,C1s XPS)和 G 带(拉曼)相对于Mg-rGO 对 H 的吸收。有趣的是,Mg-Ni-rGO 表现出 rGO 和 Ni 添加的单独效应。来自金属 Ni 和可能来自 Mg 2 NiH x的 Ni-C 相互作用导致 Mg-Ni-rGO 优异的 H 释放。这项工作证明了添加多种催化剂对 H 释放的协同作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号