当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein–protein interaction based substrate control in the E. coli octanoic acid transferase, LipB
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2021-07-28 , DOI: 10.1039/d1cb00125f Thomas G Bartholow 1 , Terra Sztain 1 , Megan A Young 1 , Tony D Davis 1 , Ruben Abagyan 2 , Michael D Burkart 1
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2021-07-28 , DOI: 10.1039/d1cb00125f Thomas G Bartholow 1 , Terra Sztain 1 , Megan A Young 1 , Tony D Davis 1 , Ruben Abagyan 2 , Michael D Burkart 1
Affiliation
|
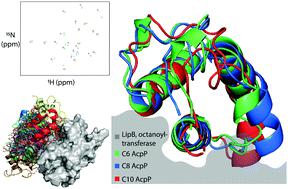
Lipoic acid is an essential cofactor produced in all organisms by diverting octanoic acid derived as an intermediate of type II fatty acid biosynthesis. In bacteria, octanoic acid is transferred from the acyl carrier protein (ACP) to the lipoylated target protein by the octanoyltransferase LipB. LipB has a well-documented substrate selectivity, indicating a mechanism of octanoic acid recognition. The present study reveals the precise protein–protein interactions (PPIs) responsible for this selectivity in Escherichia coli through a combination of solution-state protein NMR titration with high-resolution docking of the experimentally examined substrates. We examine the structural changes of substrate-bound ACP and determine the precise geometry of the LipB interface. Thermodynamic effects from varying substrates were observed by NMR, and steric occlusion of docked models indicates how LipB interprets proper substrate identity via allosteric binding. This study provides a model for elucidating how substrate identity is transferred through the ACP structure to regulate activity in octanoyl transferases.
中文翻译:

大肠杆菌辛酸转移酶 LipB 中基于蛋白质-蛋白质相互作用的底物控制
硫辛酸是所有生物体中通过转移作为 II 型脂肪酸生物合成中间体衍生的辛酸而产生的必需辅助因子。在细菌中,辛酸通过辛酰基转移酶 LipB 从酰基载体蛋白 (ACP) 转移至硫酰化靶蛋白。LipB 具有充分记录的底物选择性,表明辛酸识别机制。本研究通过溶液态蛋白质 NMR 滴定与实验检测底物的高分辨率对接相结合,揭示了大肠杆菌中造成这种选择性的精确蛋白质-蛋白质相互作用 (PPI)。我们检查了底物结合 ACP 的结构变化并确定了 LipB 界面的精确几何形状。通过 NMR 观察到不同底物的热力学效应,对接模型的空间闭塞表明 LipB 如何通过变构结合解释正确的底物身份。这项研究提供了一个模型,用于阐明底物身份如何通过 ACP 结构转移以调节辛酰基转移酶的活性。
更新日期:2021-08-09
中文翻译:

大肠杆菌辛酸转移酶 LipB 中基于蛋白质-蛋白质相互作用的底物控制
硫辛酸是所有生物体中通过转移作为 II 型脂肪酸生物合成中间体衍生的辛酸而产生的必需辅助因子。在细菌中,辛酸通过辛酰基转移酶 LipB 从酰基载体蛋白 (ACP) 转移至硫酰化靶蛋白。LipB 具有充分记录的底物选择性,表明辛酸识别机制。本研究通过溶液态蛋白质 NMR 滴定与实验检测底物的高分辨率对接相结合,揭示了大肠杆菌中造成这种选择性的精确蛋白质-蛋白质相互作用 (PPI)。我们检查了底物结合 ACP 的结构变化并确定了 LipB 界面的精确几何形状。通过 NMR 观察到不同底物的热力学效应,对接模型的空间闭塞表明 LipB 如何通过变构结合解释正确的底物身份。这项研究提供了一个模型,用于阐明底物身份如何通过 ACP 结构转移以调节辛酰基转移酶的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号