Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-08-09 , DOI: 10.1016/j.bmc.2021.116340 Thais Cristina Mendonça Nogueira 1 , Marcus Vinicius Nora de Souza 1
|
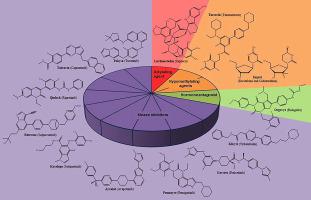
In 2020, fifty-three new drugs, including forty small-molecules (thirty-six new chemical entities and four new diagnostic agents) and thirteen biologic drugs were approved by the U.S. Food and Drug Administration (FDA). This year, small-molecules continue to play a role in innovative treatments representing around 75% of all drugs accepted by FDA. The dominant therapeutic area was oncology, accounting for twenty-three new approvals, including thirteen new chemical entities, four new diagnostic agents, and thirteen biologic drugs. Recognizing the importance of small-molecules on cancer treatment, this review aims to provide an overview regarding the clinical applications and mechanism of action of the thirteen new small-molecules (excluding new diagnostic agents) approved by FDA in 2020.
中文翻译:

2020年FDA肿瘤小分子新药获批:作用机制及临床应用
2020年,美国食品药品监督管理局(FDA)批准了53个新药,其中包括40个小分子(36个新化学实体和4个新诊断剂)和13个生物药。今年,小分子继续在创新治疗中发挥作用,约占 FDA 接受的所有药物的 75%。占主导地位的治疗领域是肿瘤学,新批准了 23 个,包括 13 个新化学实体、4 个新诊断药物和 13 个生物药物。认识到小分子在癌症治疗中的重要性,本综述旨在概述 FDA 2020 年批准的 13 种新小分子(不包括新诊断剂)的临床应用和作用机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号