当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In vivo cleavage of solubility tags as a tool to enhance the levels of soluble recombinant proteins in Escherichia coli
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2021-08-09 , DOI: 10.1002/bit.27912 Filipe S R Silva 1 , Sara P O Santos 1 , Roberto Meyer 1, 2 , Eduardo S Silva 1, 2 , Carina S Pinheiro 1, 2 , Neuza M Alcantara-Neves 1, 2 , Luis G C Pacheco 1, 3
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2021-08-09 , DOI: 10.1002/bit.27912 Filipe S R Silva 1 , Sara P O Santos 1 , Roberto Meyer 1, 2 , Eduardo S Silva 1, 2 , Carina S Pinheiro 1, 2 , Neuza M Alcantara-Neves 1, 2 , Luis G C Pacheco 1, 3
Affiliation
|
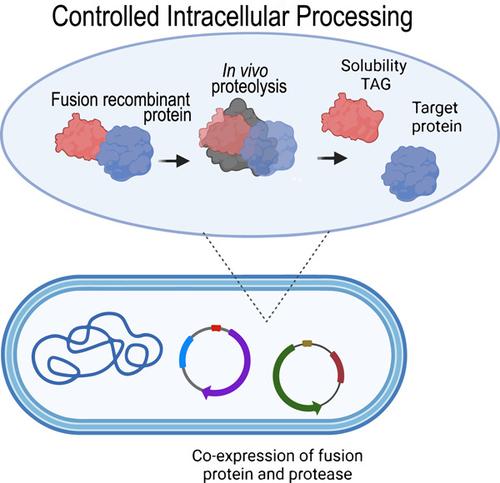
Recombinant proteins are generally fused with solubility enhancer tags to improve the folding and solubility of the target protein of interest. However, the fusion protein strategy usually requires expensive proteases to perform in vitro proteolysis and additional chromatographic steps to obtain tag-free recombinant proteins. Expression systems based on intracellular processing of solubility tags in Escherichia coli, through co-expression of a site-specific protease, simplify the recombinant protein purification process, and promote the screening of molecules that fail to remain soluble after tag removal. High yields of soluble target proteins have already been achieved using these protease co-expression systems. Herein, we review approaches for controlled intracellular processing systems tailored to produce soluble untagged proteins in E. coli. We discuss the different genetic systems available for intracellular processing of recombinant proteins regarding system design features, advantages, and limitations of the various strategies.
中文翻译:

溶解性标签的体内切割作为提高大肠杆菌中可溶性重组蛋白水平的工具
重组蛋白通常与溶解度增强剂标签融合,以改善目的靶蛋白的折叠和溶解度。然而,融合蛋白策略通常需要昂贵的蛋白酶来进行体外蛋白水解和额外的色谱步骤以获得无标签的重组蛋白。基于大肠杆菌溶解性标签细胞内加工的表达系统,通过共表达位点特异性蛋白酶,简化重组蛋白纯化过程,促进筛选去除标签后无法保持可溶性的分子。使用这些蛋白酶共表达系统已经实现了高产量的可溶性靶蛋白。在此,我们回顾了用于在大肠杆菌中生产可溶性未标记蛋白质的受控细胞内处理系统的方法。我们讨论了可用于重组蛋白细胞内加工的不同遗传系统,涉及系统设计特征、优点和各种策略的局限性。
更新日期:2021-10-13
中文翻译:

溶解性标签的体内切割作为提高大肠杆菌中可溶性重组蛋白水平的工具
重组蛋白通常与溶解度增强剂标签融合,以改善目的靶蛋白的折叠和溶解度。然而,融合蛋白策略通常需要昂贵的蛋白酶来进行体外蛋白水解和额外的色谱步骤以获得无标签的重组蛋白。基于大肠杆菌溶解性标签细胞内加工的表达系统,通过共表达位点特异性蛋白酶,简化重组蛋白纯化过程,促进筛选去除标签后无法保持可溶性的分子。使用这些蛋白酶共表达系统已经实现了高产量的可溶性靶蛋白。在此,我们回顾了用于在大肠杆菌中生产可溶性未标记蛋白质的受控细胞内处理系统的方法。我们讨论了可用于重组蛋白细胞内加工的不同遗传系统,涉及系统设计特征、优点和各种策略的局限性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号