当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Pertyolides A, B, and C: A Synthetic Procedure to C17-Sesquiterpenoids and a Study of Their Phytotoxic Activity
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-08-09 , DOI: 10.1021/acs.jnatprod.1c00396 David M Cárdenas 1 , Carlos Rial 1 , Rosa M Varela 1 , José M G Molinillo 1 , Francisco A Macías 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-08-09 , DOI: 10.1021/acs.jnatprod.1c00396 David M Cárdenas 1 , Carlos Rial 1 , Rosa M Varela 1 , José M G Molinillo 1 , Francisco A Macías 1
Affiliation
|
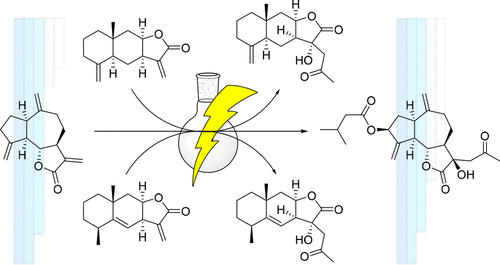
C17-sesquiterpenoids are a group of natural products that have been recently discovered. These compounds have the peculiarity of lacking the α,β-methylene butyrolactone system, which is known to be quite relevant for many of the biological activities reported for sesquiterpene lactones. Unfortunately, the biological interest of C17-sesquiterpenoids has not been studied in-depth, mainly due to the poor isolation yields in which they can be obtained from natural sources. Therefore, in order to allow a deeper study of these novel molecules, we have worked out a synthetic pathway that provides C17-sesquiterpenoids in enough quantities from easily accessible sesquiterpene lactones to enable a more thorough investigation of their bioactivities. With this synthesis method, we have successfully synthesized, for the first time, three natural C17-sesquiterpenoids, pertyolides A, B, and C, with good overall yields. Furthermore, we have also evaluated their phytotoxicity against etiolated wheat coleoptiles and corroborated that pertyolides B and C present strong phytotoxic activity.
中文翻译:

Pertyolides A、B 和 C 的合成:C17-倍半萜类化合物的合成方法及其植物毒性活性研究
C 17 -倍半萜类化合物是最近发现的一组天然产物。这些化合物的特点是缺乏 α,β-亚甲基丁内酯系统,众所周知,该系统与倍半萜内酯的许多生物活性非常相关。不幸的是,C 17 -倍半萜类化合物的生物学意义尚未得到深入研究,这主要是因为它们可以从天然来源获得的分离产率很低。因此,为了对这些新分子进行更深入的研究,我们制定了一条合成途径,提供 C 17- 从易于获得的倍半萜内酯中提取足够数量的倍半萜类化合物,以便对其生物活性进行更彻底的研究。使用这种合成方法,我们首次成功合成了三种天然C 17 -倍半萜类化合物pertyolides A、B 和C,并具有良好的总收率。此外,我们还评估了它们对黄化小麦胚芽鞘的植物毒性,并证实了 pertyolides B 和 C 具有很强的植物毒性活性。
更新日期:2021-08-27
中文翻译:

Pertyolides A、B 和 C 的合成:C17-倍半萜类化合物的合成方法及其植物毒性活性研究
C 17 -倍半萜类化合物是最近发现的一组天然产物。这些化合物的特点是缺乏 α,β-亚甲基丁内酯系统,众所周知,该系统与倍半萜内酯的许多生物活性非常相关。不幸的是,C 17 -倍半萜类化合物的生物学意义尚未得到深入研究,这主要是因为它们可以从天然来源获得的分离产率很低。因此,为了对这些新分子进行更深入的研究,我们制定了一条合成途径,提供 C 17- 从易于获得的倍半萜内酯中提取足够数量的倍半萜类化合物,以便对其生物活性进行更彻底的研究。使用这种合成方法,我们首次成功合成了三种天然C 17 -倍半萜类化合物pertyolides A、B 和C,并具有良好的总收率。此外,我们还评估了它们对黄化小麦胚芽鞘的植物毒性,并证实了 pertyolides B 和 C 具有很强的植物毒性活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号