Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2021-08-08 , DOI: 10.1016/j.yjmcc.2021.08.002 Mitchell Simon 1 , Christopher Y Ko 1 , Robyn T Rebbeck 2 , Sonya Baidar 1 , Razvan L Cornea 2 , Donald M Bers 1
|
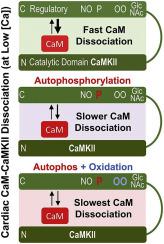
Persistent over-activation of CaMKII (Calcium/Calmodulin-dependent protein Kinase II) in the heart is implicated in arrhythmias, heart failure, pathological remodeling, and other cardiovascular diseases. Several post-translational modifications (PTMs)—including autophosphorylation, oxidation, S-nitrosylation, and O-GlcNAcylation—have been shown to trap CaMKII in an autonomously active state. The molecular mechanisms by which these PTMs regulate calmodulin (CaM) binding to CaMKIIδ—the primary cardiac isoform—has not been well-studied particularly in its native myocyte environment.
Typically, CaMKII activates upon Ca-CaM binding during locally elevated [Ca]free and deactivates upon Ca-CaM dissociation when [Ca]free returns to basal levels. To assess the effects of CaMKIIδ PTMs on CaM binding, we developed a novel FRET (Förster resonance energy transfer) approach to directly measure CaM binding to and dissociation from CaMKIIδ in live cardiac myocytes. We demonstrate that autophosphorylation of CaMKIIδ increases affinity for CaM in its native environment and that this increase is dependent on [Ca]free. This leads to a 3-fold slowing of CaM dissociation from CaMKIIδ (time constant slows from ~0.5 to 1.5 s) when [Ca]free is reduced with physiological kinetics. Moreover, oxidation further slows CaM dissociation from CaMKIIδ T287D (phosphomimetic) upon rapid [Ca]free chelation and increases FRET between CaM and CaMKIIδ T287A (phosphoresistant).
The CaM dissociation kinetics–measured here in myocytes–are similar to the interval between heartbeats, and integrative memory would be expected as a function of heart rate. Furthermore, the PTM-induced slowing of dissociation between beats would greatly promote persistent CaMKIIδ activity in the heart. Together, these findings suggest a significant role of PTM-induced changes in CaMKIIδ affinity for CaM and memory under physiological and pathophysiological processes in the heart.
中文翻译:

CaMKIIδ 翻译后修饰增加了对心室肌细胞内钙调蛋白的亲和力
心脏中 CaMKII(钙/钙调蛋白依赖性蛋白激酶 II)的持续过度激活与心律失常、心力衰竭、病理性重塑和其他心血管疾病有关。几种翻译后修饰 (PTM)——包括自磷酸化、氧化、S-亚硝基化和O -GlcNAcylation——已被证明可以将 CaMKII 捕获在自主活性状态。这些 PTM 调节钙调蛋白 (CaM) 与 CaMKIIδ(主要心脏同种型)结合的分子机制尚未得到充分研究,特别是在其天然肌细胞环境中。
通常,CaMKII 在局部升高的 [Ca]游离期间在 Ca-CaM 结合时激活,并在 [Ca]游离恢复到基础水平时在 Ca-CaM 解离时失活。为了评估 CaMKIIδ PTM 对 CaM 结合的影响,我们开发了一种新的 FRET(Förster 共振能量转移)方法来直接测量活心肌细胞中 CaM 与 CaMKIIδ 的结合和解离。我们证明 CaMKIIδ 的自磷酸化增加了在其原生环境中对 CaM 的亲和力,并且这种增加依赖于 [Ca]游离。当 [Ca]游离时,这导致 CaM 与 CaMKIIδ 的解离减慢 3 倍(时间常数从 ~0.5 减至 1.5 s)随生理动力学而降低。此外,在快速无[Ca] 螯合时,氧化会进一步减慢 CaM 从 CaMKIIδ T287D(磷模拟物)的解离,并增加 CaM 和 CaMKIIδ T287A(抗磷)之间的 FRET。
在这里测量的肌细胞中的 CaM 解离动力学类似于心跳之间的间隔,并且预计综合记忆是心率的函数。此外,PTM 诱导的节拍间解离减慢将极大地促进心脏中持续的 CaMKIIδ 活性。总之,这些发现表明 PTM 诱导的 CaMKIIδ 亲和力变化在心脏生理和病理生理过程下对 CaM 和记忆具有重要作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号