Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2021-08-08 , DOI: 10.1016/j.apcata.2021.118309 Christopher R. Riley 1 , Andrew De La Riva 2 , Isabel L. Ibarra 2 , Abhaya K. Datye 2 , Stanley S. Chou 1
|
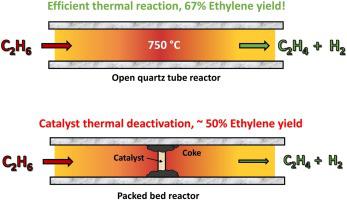
Steam cracking of ethane, a non-catalytic thermochemical process, remains the dominant means of ethylene production. The severe reaction conditions and energy expenditure involved in this process incentivize the search for alternative reaction pathways and reactor designs which maximize ethylene yield while minimizing cost and energy input. Herein, we report a comparison of catalytic and non-catalytic non-oxidative dehydrogenation of ethane. We achieve ethylene yields as high as 67 % with an open tube quartz reactor without the use of a catalyst at residence times ∼4 s. The open tube reactor design promotes simplicity, low cost, and negligible coke formation. Pristine quartz tubes were most effective, since coke formation was detected when defects were introduced by scratching the surface of the quartz. Surprisingly, the addition of solids to the quartz tube, such as quartz sand, alumina powder, or even Pt-based intermetallic catalysts, led to lower ethylene yield. Pt alloy catalysts are effective at lower temperatures, such as at 575 °C, but conversion is limited due to thermodynamic constraints. When operated at industrially relevant temperatures, such as 700 °C and above, these catalysts were not stable in our tests, causing ethylene yield to drop below that of the open tube. These results suggest that future research on non-oxidative dehydrogenation should be directed at optimizing reactor designs to improve the conversion of ethane to ethylene, since this approach shows promise for decentralized production of ethylene from natural gas deposits.
中文翻译:

在非氧化乙烷脱氢中实现高乙烯收率
乙烷的蒸汽裂解是一种非催化热化学过程,仍然是乙烯生产的主要手段。该过程中涉及的严酷反应条件和能源消耗促使人们寻找替代反应途径和反应器设计,以最大限度地提高乙烯产量,同时最大限度地降低成本和能源输入。在此,我们报告了乙烷的催化和非催化非氧化脱氢的比较。我们在不使用催化剂的情况下使用开管石英反应器实现了高达 67% 的乙烯产率,停留时间约为 4 秒。开管反应器设计促进了简单性、低成本和可忽略的焦炭形成。原始石英管是最有效的,因为当通过刮擦石英表面引入缺陷时可以检测到焦炭的形成。出奇,向石英管中加入固体,如石英砂、氧化铝粉,甚至铂基金属间化合物催化剂,导致乙烯收率降低。Pt 合金催化剂在较低温度下有效,例如在 575°C,但由于热力学限制,转化率受到限制。当在工业相关温度(例如 700 °C 及更高)下操作时,这些催化剂在我们的测试中不稳定,导致乙烯产率低于开管的产率。这些结果表明,未来对非氧化脱氢的研究应着眼于优化反应器设计,以提高乙烷向乙烯的转化率,因为这种方法显示了从天然气矿床分散生产乙烯的前景。导致乙烯收率降低。Pt 合金催化剂在较低温度下有效,例如在 575°C,但由于热力学限制,转化率受到限制。当在工业相关温度(例如 700 °C 及更高)下操作时,这些催化剂在我们的测试中不稳定,导致乙烯产率低于开管的产率。这些结果表明,未来对非氧化脱氢的研究应着眼于优化反应器设计,以提高乙烷向乙烯的转化率,因为这种方法显示了从天然气矿床分散生产乙烯的前景。导致乙烯收率降低。Pt 合金催化剂在较低温度下有效,例如在 575°C,但由于热力学限制,转化率受到限制。当在工业相关温度(例如 700 °C 及更高)下操作时,这些催化剂在我们的测试中不稳定,导致乙烯产率低于开管的产率。这些结果表明,未来对非氧化脱氢的研究应着眼于优化反应器设计,以提高乙烷向乙烯的转化率,因为这种方法显示了从天然气矿床分散生产乙烯的前景。这些催化剂在我们的测试中不稳定,导致乙烯产率低于开管的产率。这些结果表明,未来对非氧化脱氢的研究应着眼于优化反应器设计,以提高乙烷向乙烯的转化率,因为这种方法显示了从天然气矿床分散生产乙烯的前景。这些催化剂在我们的测试中不稳定,导致乙烯产率低于开管的产率。这些结果表明,未来对非氧化脱氢的研究应着眼于优化反应器设计,以提高乙烷向乙烯的转化率,因为这种方法显示了从天然气矿床分散生产乙烯的前景。









































 京公网安备 11010802027423号
京公网安备 11010802027423号