Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2021-08-08 , DOI: 10.1016/j.enzmictec.2021.109881 Siyu Ren 1 , Xinkuan Cheng 1 , Long Ma 1
|
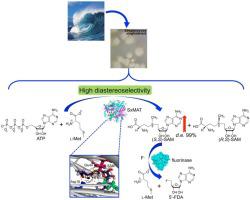
Natural fluorinated products are rare and attract great attention. The de novo fluorometabolites biosynthetic pathway in microbes has been studied. It is revealed that the carbon-fluorine (C-F) bond is formed by an exotic enzyme called fluorinase (FLA) when using fluorine ions and S-adenosyl-l-methionine (SAM) as substrates. However, the resource of the precursor SAM is still elusive. To solve this, a novel methionine adenosyltransferase from Streptomyces xinghaiensis (SxMAT) was identified and characterized. We proved that SAM was enzymatically synthesized by SxMAT, an enzyme that mediated the reaction between adenosine triphosphate (ATP) and l-methionine (l-Met) with 99% diastereoisomeric excess (d.e.) and 80% yield. Such high diastereoselectivity had never been reported before. SxMAT was a Co2+-dependent metalloenzyme. The results showed that the metal cobalt ion contributes to the activity and selectivity of SxMAT. Molecular docking was performed to reveal its catalytic mechanism. The optimal temperature and pH were 55 °C and 8.5, respectively. Lastly, a two-step tandem enzymatic reaction using SxMAT and FLA both from S. xinghaiensis to generate 5’-fluoro-deoxyadenosine (5’-FDA) was performed. This implied that SxMAT may be present in this fluorometabolites biosynthetic route. These results suggested that SxMAT could be a useful biocatalyst for the synthesis of optically pure (S)-S-adenosyl-l-methionine, an important nutraceutical. In addition, SxMAT will probably play an important role in the biosynthetic pathway of fluorinated natural products in bacteria.
中文翻译:

鉴定具有高非对映选择性的甲硫氨酸腺苷转移酶生物催化合成(S)-S-腺苷-l-甲硫氨酸及其与氟化生物合成途径的关系
天然含氟产品稀有,备受关注。该从头fluorometabolites生物合成途径中的微生物进行了研究。研究表明,当使用氟离子和S-腺苷-l-甲硫氨酸 (SAM) 作为底物时,碳-氟 (CF) 键是由一种称为氟化酶 (FLA) 的外来酶形成的。然而,前体SAM的资源仍然难以捉摸。为了解决这个问题,鉴定并表征了一种来自星海链霉菌(SxMAT)的新型甲硫氨酸腺苷转移酶。我们证明 SAM 是由 SxMAT 酶促合成的,SxMAT 是一种介导三磷酸腺苷 (ATP) 和l-蛋氨酸 ( l-Met) 具有 99% 的非对映异构体过量 ( de ) 和 80% 的产率。以前从未报道过如此高的非对映选择性。SxMAT 是一种 Co 2+依赖性金属酶。结果表明,金属钴离子有助于提高 SxMAT 的活性和选择性。进行分子对接以揭示其催化机制。最适温度和 pH 值分别为 55 °C 和 8.5。最后,使用SxMAT和FLA无论从两步串联酶促反应小号。xinghaiensis生成 5'-氟脱氧腺苷 (5'-FDA)。这意味着 SxMAT 可能存在于这种含氟代谢物的生物合成途径中。这些结果表明 SxMAT 可能是一种有用的生物催化剂,用于合成光学纯(S )-S-腺苷-l-甲硫氨酸,一种重要的营养品。此外,SxMAT 可能在细菌中氟化天然产物的生物合成途径中发挥重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号