当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mutations in the binding site of TNFR1 PLAD reduce homologous interactions but can enhance antagonism of wild-type TNFR1 activity
Immunology ( IF 4.9 ) Pub Date : 2021-08-07 , DOI: 10.1111/imm.13400 Sarah Albogami 1, 2 , Ian Todd 1 , Ola Negm 1, 3 , Lucy C Fairclough 1 , Patrick J Tighe 1
Immunology ( IF 4.9 ) Pub Date : 2021-08-07 , DOI: 10.1111/imm.13400 Sarah Albogami 1, 2 , Ian Todd 1 , Ola Negm 1, 3 , Lucy C Fairclough 1 , Patrick J Tighe 1
Affiliation
|
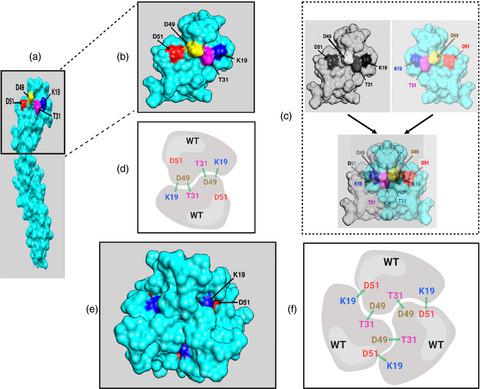
The tumour necrosis factor receptor superfamily (TNFRSF) members contain cysteine-rich domains (CRD) in their extracellular regions, and the membrane-distal CRD1 forms homologous interactions in the absence of ligand. The CRD1 is therefore termed a pre-ligand assembly domain (PLAD). The role of PLAD-PLAD interactions in the induction of signalling as a consequence of TNF-TNFR binding led to the development of soluble PLAD domains as antagonists of TNFR activation. In the present study, we generated recombinant wild-type (WT) PLAD of TNFR1 and mutant forms with single alanine substitutions of amino acid residues thought to be critical for the formation of homologous dimers and/or trimers of PLAD (K19A, T31A, D49A and D52A). These mutated PLADs were compared with WT PLAD as antagonists of TNF-induced apoptosis or the activation of inflammatory signalling pathways. Unlike WT PLAD, the mutated PLADs showed little or no homologous interactions, confirming the importance of particular amino acids as contact residues in the PLAD binding region. However, as with WT PLAD, the mutated PLADs functioned as antagonists of TNF-induced TNFR1 activity leading to induction of cell death or NF-κB signalling. Indeed, some of the mutant PLADs, and K19A PLAD in particular, showed enhanced antagonistic activity compared with WT PLAD. This is consistent with the reduced formation of homologous multimers by these PLAD mutants effectively increasing the concentration of PLAD available to bind and antagonize WT TNFR1 when compared to WT PLAD acting as an antagonist. This may have implications for the development of antagonistic PLADs as therapeutic agents.
中文翻译:

TNFR1 PLAD 结合位点的突变减少了同源相互作用,但可以增强对野生型 TNFR1 活性的拮抗作用
肿瘤坏死因子受体超家族 (TNFRSF) 成员在其细胞外区域包含富含半胱氨酸的结构域 (CRD),并且膜远端 CRD1 在没有配体的情况下形成同源相互作用。因此,CRD1 被称为前配体组装结构域 (PLAD)。由于 TNF-TNFR 结合,PLAD-PLAD 相互作用在信号诱导中的作用导致可溶性 PLAD 结构域作为 TNFR 激活的拮抗剂的发展。在本研究中,我们产生了 TNFR1 的重组野生型 (WT) PLAD 和突变形式,其中氨基酸残基的单个丙氨酸取代被认为对 PLAD 的同源二聚体和/或三聚体的形成至关重要(K19A、T31A、D49A和 D52A)。这些突变的 PLAD 与 WT PLAD 作为 TNF 诱导的细胞凋亡或炎症信号通路激活的拮抗剂进行了比较。与 WT PLAD 不同,突变的 PLAD 显示很少或没有同源相互作用,证实了特定氨基酸作为 PLAD 结合区域中接触残基的重要性。然而,与 WT PLAD 一样,突变的 PLAD 作为 TNF 诱导的 TNFR1 活性的拮抗剂,导致细胞死亡或 NF-κB 信号传导的诱导。事实上,与 WT PLAD 相比,一些突变的 PLAD,特别是 K19A PLAD,显示出增强的拮抗活性。这与这些 PLAD 突变体减少同源多聚体的形成一致,与作为拮抗剂的 WT PLAD 相比,有效地增加了可用于结合和拮抗 WT TNFR1 的 PLAD 浓度。
更新日期:2021-10-15
中文翻译:

TNFR1 PLAD 结合位点的突变减少了同源相互作用,但可以增强对野生型 TNFR1 活性的拮抗作用
肿瘤坏死因子受体超家族 (TNFRSF) 成员在其细胞外区域包含富含半胱氨酸的结构域 (CRD),并且膜远端 CRD1 在没有配体的情况下形成同源相互作用。因此,CRD1 被称为前配体组装结构域 (PLAD)。由于 TNF-TNFR 结合,PLAD-PLAD 相互作用在信号诱导中的作用导致可溶性 PLAD 结构域作为 TNFR 激活的拮抗剂的发展。在本研究中,我们产生了 TNFR1 的重组野生型 (WT) PLAD 和突变形式,其中氨基酸残基的单个丙氨酸取代被认为对 PLAD 的同源二聚体和/或三聚体的形成至关重要(K19A、T31A、D49A和 D52A)。这些突变的 PLAD 与 WT PLAD 作为 TNF 诱导的细胞凋亡或炎症信号通路激活的拮抗剂进行了比较。与 WT PLAD 不同,突变的 PLAD 显示很少或没有同源相互作用,证实了特定氨基酸作为 PLAD 结合区域中接触残基的重要性。然而,与 WT PLAD 一样,突变的 PLAD 作为 TNF 诱导的 TNFR1 活性的拮抗剂,导致细胞死亡或 NF-κB 信号传导的诱导。事实上,与 WT PLAD 相比,一些突变的 PLAD,特别是 K19A PLAD,显示出增强的拮抗活性。这与这些 PLAD 突变体减少同源多聚体的形成一致,与作为拮抗剂的 WT PLAD 相比,有效地增加了可用于结合和拮抗 WT TNFR1 的 PLAD 浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号