Materials Today Chemistry ( IF 6.7 ) Pub Date : 2021-08-07 , DOI: 10.1016/j.mtchem.2021.100533 R. Ediati 1 , W. Aulia 1 , B.A. Nikmatin 1 , A.R.P. Hidayat 1 , U.M. Fitriana 1 , C. Muarifah 1 , D.O. Sulistiono 1 , F. Martak 1 , D. Prasetyoko 1
|
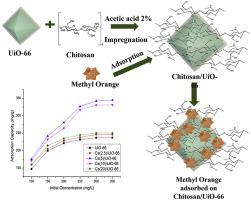
UiO-66 and chitosan/UiO-66 composites were successfully synthesized by varying the mass addition of chitosan which were 0%, 2.5%, 5%, 10%, and 20% of the mass of UiO-66, denoted as UiO-66, Cs(2.5)/UiO-66, Cs(5)/UiO-66, Cs(10)/UiO-66, and Cs(20)/UiO-66, respectively. UiO-66 was modified with chitosan using the impregnation process. The X-ray diffraction patterns of the synthesized materials showed characteristic peaks at 2θ of 7.25° and 8.39°, which matched to that of the reported UiO-66. In addition, the Fourier transform infrared spectroscopy spectra of the materials showed absorption bands at the same wavenumber as UiO-66 and chitosan previously reported. The surface morphology of UiO-66 observed from scanning electron microscopy images was in the form of agglomerated small cube particles, where the smaller particles were observed for Cs(10)/UiO-66. From the N2 adsorption isotherms, it was found that the Brunauer-Emmett-Teller surface areas of UiO-66, Cs(5)/UiO-66, and Cs(10)/UiO-66 materials were 825.7 m2/g, 835.4 m2/g, and 882.2 m2/g, respectively. The results of the study on adsorption of methyl orange in aqueous solutions showed that Cs(5)/UiO-66 had the highest adsorption capacity of 370.37 mg/g and followed the pseudo–second-order adsorption kinetic with a Langmuir isotherm model.
中文翻译:

壳聚糖/UiO-66复合材料作为高性能吸附剂去除水溶液中的甲基橙
UiO-66和壳聚糖/UiO-66复合材料通过改变壳聚糖的质量添加量成功合成,壳聚糖的质量分别为UiO-66质量的0%、2.5%、5%、10%和20%,记为UiO-66 、Cs(2.5)/UiO-66、Cs(5)/UiO-66、Cs(10)/UiO-66 和 Cs(20)/UiO-66。UiO-66 使用浸渍工艺用壳聚糖改性。合成材料的 X 射线衍射图显示 2θ 为 7.25° 和 8.39° 的特征峰,与报道的 UiO-66 相匹配。此外,材料的傅里叶变换红外光谱显示出与之前报道的 UiO-66 和壳聚糖相同波数的吸收带。从扫描电镜图像中观察到的 UiO-66 的表面形态为团聚的小立方体颗粒,其中对于 Cs(10)/UiO-66 观察到较小的颗粒。从那时起2吸附等温线,发现UiO-66、Cs(5)/UiO-66和Cs(10)/UiO-66材料的Brunauer-Emmett-Teller表面积分别为825.7 m 2 /g、835.4 m 2 /g 和 882.2 m 2 /g。甲基橙在水溶液中的吸附研究结果表明,Cs(5)/UiO-66 的吸附容量最高,为 370.37 mg/g,并遵循 Langmuir 等温线模型的准二级吸附动力学。









































 京公网安备 11010802027423号
京公网安备 11010802027423号