当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promoted peroxymonosulfate activation by electron transport channel construction for rapid Cu(II)/Cu(I) redox couple circulation
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2021-07-24 , DOI: 10.1039/d1en00426c Ting Chen 1, 2, 3, 4, 5 , Zhiliang Zhu 1, 2, 3, 4, 5 , Yujie Bao 1, 2, 3, 4, 5 , Hua Zhang 1, 2, 3, 4 , Yanling Qiu 2, 3, 4, 5, 6 , Daqiang Yin 2, 3, 4, 5, 6
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2021-07-24 , DOI: 10.1039/d1en00426c Ting Chen 1, 2, 3, 4, 5 , Zhiliang Zhu 1, 2, 3, 4, 5 , Yujie Bao 1, 2, 3, 4, 5 , Hua Zhang 1, 2, 3, 4 , Yanling Qiu 2, 3, 4, 5, 6 , Daqiang Yin 2, 3, 4, 5, 6
Affiliation
|
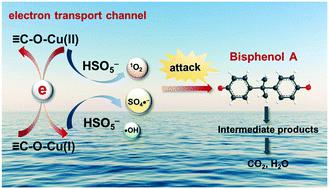
The effective construction of electron transport channels has essential significance for electron-transfer rate enhancement in REDOX processes during peroxymonosulfate (PMS) activation. This study focuses on the establishment of Cu-involved covalent bonds due to the unique electron configuration of Cu ions. The strategy was achieved by anchoring Cu atoms to an O-doped g-C3N4 (Cu–O–CN) matrix. The C–O–Cu linkage was successfully constructed, derived from the precise incorporation of oxygen. From XAFS, H2-TPR, and solid-ESR characteristic observations, the desired REDOX performance was verified. The constructed C–O–Cu linkages were treated as electron transport channels for electron transfer while reacting with PMS, effectively accelerating the REDOX circulation of the Cu(II)/Cu(I) redox couple and promoting the consecutive generation of active species. Consequently, nearly 100% bisphenol A (BPA) was degraded in the Cu–O–CN/PMS reaction system within 30 min. This demonstrates the effectiveness of Cu–O–CN for PMS activation. The prepared Cu–O–CN exhibited definite stability and potential reusability. This research not only highlights the tailored design of heterogeneous catalysts for PMS activation but also broadens its application in water purification.
中文翻译:

通过电子传输通道构建促进过硫酸盐活化以实现快速 Cu(II)/Cu(I) 氧化还原对循环
电子传输通道的有效构建对于过硫酸盐 (PMS) 活化过程中 REDOX 过程中电子传输速率的提高具有重要意义。由于铜离子独特的电子构型,本研究的重点是建立与铜有关的共价键。该策略是通过将 Cu 原子锚定到 O 掺杂的 gC 3 N 4 (Cu-O-CN) 基质上来实现的。成功地构建了 C-O-Cu 键,源于氧的精确结合。来自 XAFS,H 2-TPR 和固体 ESR 特性观察,验证了所需的 REDOX 性能。构建的 C-O-Cu 键在与 PMS 反应时被视为电子传输的电子传输通道,有效加速了 Cu( II )/Cu( I ) 氧化还原对的氧化还原循环并促进了活性物种的连续生成。因此,在 30 分钟内,Cu-O-CN/PMS 反应体系中几乎 100% 双酚 A (BPA) 被降解。这证明了 Cu-O-CN 对 PMS 活化的有效性。制备的 Cu-O-CN 表现出一定的稳定性和潜在的可重用性。这项研究不仅突出了用于 PMS 活化的多相催化剂的定制设计,而且还拓宽了其在水净化中的应用。
更新日期:2021-08-07
中文翻译:

通过电子传输通道构建促进过硫酸盐活化以实现快速 Cu(II)/Cu(I) 氧化还原对循环
电子传输通道的有效构建对于过硫酸盐 (PMS) 活化过程中 REDOX 过程中电子传输速率的提高具有重要意义。由于铜离子独特的电子构型,本研究的重点是建立与铜有关的共价键。该策略是通过将 Cu 原子锚定到 O 掺杂的 gC 3 N 4 (Cu-O-CN) 基质上来实现的。成功地构建了 C-O-Cu 键,源于氧的精确结合。来自 XAFS,H 2-TPR 和固体 ESR 特性观察,验证了所需的 REDOX 性能。构建的 C-O-Cu 键在与 PMS 反应时被视为电子传输的电子传输通道,有效加速了 Cu( II )/Cu( I ) 氧化还原对的氧化还原循环并促进了活性物种的连续生成。因此,在 30 分钟内,Cu-O-CN/PMS 反应体系中几乎 100% 双酚 A (BPA) 被降解。这证明了 Cu-O-CN 对 PMS 活化的有效性。制备的 Cu-O-CN 表现出一定的稳定性和潜在的可重用性。这项研究不仅突出了用于 PMS 活化的多相催化剂的定制设计,而且还拓宽了其在水净化中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号