当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lysine Deacetylase Substrate Selectivity: A Dynamic Ionic Interaction Specific to KDAC8
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-06 , DOI: 10.1021/acs.biochem.1c00384 Tasha B Toro 1 , Jordan S Swanier 1 , Jada A Bezue 1 , Christian G Broussard 1 , Terry J Watt 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-06 , DOI: 10.1021/acs.biochem.1c00384 Tasha B Toro 1 , Jordan S Swanier 1 , Jada A Bezue 1 , Christian G Broussard 1 , Terry J Watt 1
Affiliation
|
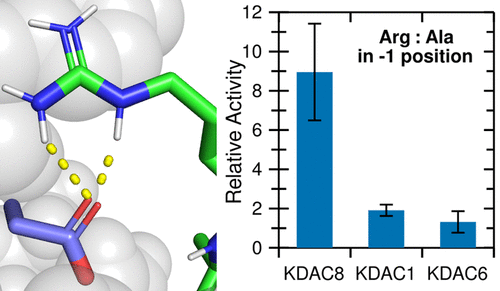
Lysine acetylation and deacetylation are critical for regulation of many cellular proteins. Despite the importance of this cycle, it is unclear how lysine deacetylase (KDAC) family members discriminate between acetylated proteins to react with a discrete set of substrates. Potential short-range interactions between KDAC8 and a known biologically relevant peptide substrate were identified using molecular dynamics (MD) simulations. Activity assays with a panel of peptides derived from this substrate supported a putative ionic interaction between arginine at the −1 substrate position and KDAC8 D101. Additional assays and MD simulations confirmed this novel interaction, which promotes deacetylation of substrates. Verification that a negatively charged residue at the 101 position is necessary for the ionic interaction and observed reactivity with the substrates was performed using KDAC8 derivatives. Notably, this interaction is specific to KDAC8, as KDAC1 and KDAC6 do not form this interaction and each KDAC has a different specificity profile with the peptide substrates, even though all KDACs could potentially form ionic interactions. When reacted with a panel of putative human KDAC substrates, KDAC8 preferentially deacetylated substrates containing an arginine at the −1 position. KDAC8 D101-R(−1) is a specific enzyme–substrate interaction that begins to explain how KDACs discriminate between potential substrates and how different KDAC family members can react with different subsets of acetylated proteins in cells. This multi-pronged approach will be extended to identify other critical interactions for KDAC8 substrate binding and determine critical interactions for other KDACs.
中文翻译:

赖氨酸脱乙酰酶底物选择性:KDAC8 特有的动态离子相互作用
赖氨酸乙酰化和去乙酰化对于许多细胞蛋白的调节至关重要。尽管这个循环很重要,但尚不清楚赖氨酸脱乙酰酶 (KDAC) 家族成员如何区分乙酰化蛋白质以与一组离散的底物反应。使用分子动力学 (MD) 模拟确定了 KDAC8 和已知的生物学相关肽底物之间潜在的短程相互作用。使用源自该底物的一组肽进行的活性测定支持在-1 底物位置的精氨酸与 KDAC8 D101 之间推定的离子相互作用。额外的分析和 MD 模拟证实了这种新的相互作用,它促进了底物的脱乙酰化。使用 KDAC8 衍生物验证 101 位带负电的残基对于离子相互作用和观察到的与底物的反应性是必需的。值得注意的是,这种相互作用是 KDAC8 特有的,因为 KDAC1 和 KDAC6 不形成这种相互作用,并且每个 KDAC 对肽底物具有不同的特异性特征,即使所有 KDAC 都可能形成离子相互作用。当与一组推定的人类 KDAC 底物反应时,KDAC8 优先使在 -1 位含有精氨酸的底物脱乙酰化。KDAC8 D101-R(-1) 是一种特定的酶-底物相互作用,它开始解释 KDAC 如何区分潜在的底物以及不同的 KDAC 家族成员如何与细胞中不同的乙酰化蛋白亚群发生反应。
更新日期:2021-08-24
中文翻译:

赖氨酸脱乙酰酶底物选择性:KDAC8 特有的动态离子相互作用
赖氨酸乙酰化和去乙酰化对于许多细胞蛋白的调节至关重要。尽管这个循环很重要,但尚不清楚赖氨酸脱乙酰酶 (KDAC) 家族成员如何区分乙酰化蛋白质以与一组离散的底物反应。使用分子动力学 (MD) 模拟确定了 KDAC8 和已知的生物学相关肽底物之间潜在的短程相互作用。使用源自该底物的一组肽进行的活性测定支持在-1 底物位置的精氨酸与 KDAC8 D101 之间推定的离子相互作用。额外的分析和 MD 模拟证实了这种新的相互作用,它促进了底物的脱乙酰化。使用 KDAC8 衍生物验证 101 位带负电的残基对于离子相互作用和观察到的与底物的反应性是必需的。值得注意的是,这种相互作用是 KDAC8 特有的,因为 KDAC1 和 KDAC6 不形成这种相互作用,并且每个 KDAC 对肽底物具有不同的特异性特征,即使所有 KDAC 都可能形成离子相互作用。当与一组推定的人类 KDAC 底物反应时,KDAC8 优先使在 -1 位含有精氨酸的底物脱乙酰化。KDAC8 D101-R(-1) 是一种特定的酶-底物相互作用,它开始解释 KDAC 如何区分潜在的底物以及不同的 KDAC 家族成员如何与细胞中不同的乙酰化蛋白亚群发生反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号